My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.25 n.1 Madrid Jan./Feb. 2010
Effect of etoricoxib, a cyclooxygenase-2 selective inhibitor on aberrant crypt formation and apoptosis in 1,2 dimethyl hydrazine induced colon carcinogenesis in rat model
Efecto del etoricoxib, un inhibidor selectivo de la ciclooxigenasa-2, sobre la formación de criptas aberrantes y la apoptosis en un modelo murino de carcinogénesis de colon inducidad por 1,2-dimetilhidracina
P. Sharma, J. Kaur and S. N. Sanyal
Department of Biophysics. Panjab University. Chandigarh-160014. India.
Financial assistance from the University Grants Commission, Major Research Project [32-614/2006 (SR)] and University Grants Commission (UGC) - Special Assistance Programme [F.3-13/2004 (SAP-II)] are gratefully acknowledged.
ABSTRACT
Etoricoxib, a second generation selective cyclooxygenase-2 (COX-2) inhibitor had been studied for the chemopreventive response at its therapeutic anti-inflammatory dose in 1,2-dimethylhydrazine (DMH) induced colon carcinogenesis in rat model. Eight to ten weeks old male rats of Sprague-Dawley strain were divided into four groups. While group 1 served as control and received the vehicle of the drugs, group 2 and 3 were administered freshly prepared DMH in 1mM EDTA-saline (pH 7.0) (30 mg/kg body wt/week, subcutaneously). Group 3 was also given a daily treatment of etoricoxib (0.6 mg/kg body wt orally) while the group 4 received the same amount of etoricoxib only, prepared in 0.5% carboxymethyl cellulose. Animals were sacrificed at the end of 6 weeks, body weight recorded and the colons were subjected to macroscopic and histopathological studies. The maximum number of raised mucosal lesions called the multiple plaque lesions (MPL) were found in the DMH group which significantly reverted back in the DMH + etoricoxib group, while very few MPLs were recorded in the control and etoricoxib only group. Similarly, the number of aberrant crypt foci (ACF), the point of future carcinogenic growth, was recorded more in the DMH group and significantly less in the DMH + etoricoxib group. The histopathological analysis showed the presence of severe hyperplasia, occasional dysplasia and aggregates of lymphoid cells in the localized regions. Etoricoxib group showed near normal histological features with the crypt architecture and the surrounding stromal tissue remaining intact. To ascertain the molecular mechanism of such anti-carcinogenic features the colonocytes were isolated and studied in primary culture for the evidence of apoptosis by fluorescent staining and genotoxic changes by single cell gel electrophoresis assay (comet assay) which shows that the DMH treated animals produced much less apoptotic nuclei but more comet producing cell, while these features were reverted back with the etoricoxib treatment. The cytoplasmic expression of COX-2 protein was studied in paraffin sections of the colon by immunohistochemistry with COX-2 specific antibody which showed a very high presence of this inducible enzyme with the DMH group while in all other groups of animals it was not visible or weekly expressed. The anti-inflammatory effect of the drug, etoricoxib was also validated by a carrageenan-induced inflammation in rat model which showed an extremely high anti-inflammatory response within the dose range used in the present study. Also the growth profile of all the animals remained the same throughout the six week period of the investigation as there was no change in the body weight. It appears that apoptosis remains the dominant anti-proliferative end effect of this drug, mediated by an inhibition of the proinflammatory COX-2 isoform although further molecular probings are needed to arrive at a conclusive agreement in favor of the chemoprotective use of such drugs in colon cancers.
Key words: Etoricoxib. Non-steroidal anti-inflammatory drugs. Colon cancer. Aberrant crypt foci. COX-2 expression. Apoptosis.
RESUMEN
El Etoricoxib, un inhibidor selectivo de la ciclooxigenasa-2 (COX-2) de segunda generación, se ha estudiado por la respuesta quimiopreventiva a su dosis terapéutica antiinflamatoria en un modelo murino de carcinogénesis colónica inducida por 1,2-dimetilhidracina (DMH). Se dividió en cuatro grupos a ratas de la cepa Sprague-Dawley de ocho a diez semanas de vida. El grupo 1 sirvió de control y recibió el vehículo de los fármacos, y a los grupos 2 y 3 se les administró DMH recién preparada en EDTA-salino al 1 mM (pH 7,0) (30 mg/kg peso corporal/semana, subcutáneamente). El grupo 3 también recibió a diario tratamiento con etoricoxib (0,6 mg/kg peso corporal, por vía oral), mientras que el grupo 4 solamente recibió la misma cantidad de etoricoxib, preparado en 0,5% carboximetil celulosa. Se sacrificó a los animales al final de la sexta semana, se registró el peso corporal y se realizaron estudios macroscópicos e histopatológicos del colon. El número máximo de lesiones mucosas elevadas, denominadas lesiones en placa múltiples (LPM) se halló en el grupo DMH, y estaba significativamente normalizado en el grupo de DMH + etoricoxib, mientras que se hallaron muy pocas lesiones LPM en los grupos control y con sólo etoricoxib. De forma similar, el número de focos de criptas aberrantes (FCA), el punto del futuro crecimiento carcinogénico, se registró más abundantemente en el grupo DMH y menos significativamente en el grupo DMH + etoricoxib. El análisis histopatológico mostró la presencia de una hiperplasia marcada, displasia ocasional y agregados de células linfoides en las regiones localizadas. El grupo de etoricoxib mostró unas características histológicas casi normales, permaneciendo la arquitectura de las criptas y el tejido estromal vecino intacto. Para determinar el mecanismo molecular de tales hallazgos anticarcinogénicos, se aislaron los colonocitos y se estudiaron en un cultivo primario para evidencia de apoptosis mediante tinción fluorescente y cambios genotóxicos en un ensayo de electroforesis en gel de una única célula (ensayo comet), que mostró que los animales tratados con DMH produjeron muchos menos núcleos apoptóticos pero un mayor número de células productoras de comet, mientras que estos cambios revirtieron con el tratamiento con etoricoxib. Se estudió la expresión citoplásmica de la proteína COX-2 en las secciones en parafina del colon mediante inmunohistoquímica con un anticuerpo específico anti-COX-2 que mostró una presencia muy alta de esta enzima inducible en el grupo DMH mientras que en el resto de los grupos de animales apenas se expresó o no se visualizó. Efecto antiinflamatorio del fármaco: el etoricoxib también se validó en modelo murino de inflamación inducida por carragenina que mostró una respuesta antiinflamatoria extremadamente alta dentro del rango de dosis empleado en este estudio. El perfil de crecimiento de los animales permaneció inalterado durante las seis semanas que duró la investigación y no hubo cambios en el peso corporal. Parece que la apoptosis sigue siendo el efecto antiproliferativo final dominante de este fármaco mediado por la inhibición de la isoforma proinflamatoria de la COX-2, si bien se necesitan probandos moleculares adicionales para llegar a un acuerdo concluyente a favor del uso quimiopreventivo de tales fármacos en los cánceres de colon.
Palabras clave: Etoricoxib. Fármacos antiinflamatorios no esteroideos. Cancer de colon. Focos de criptas aberrantes. Expresión de la COX-2. Apoptosis.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the enzyme cyclooxygenase (COX), responsible for the conversion of arachidonic acid to prostaglandins (PGs) which are important mediators of signal transduction pathways involved in cellular adhesion, proliferation, growth and differentiation.1 However, in recent times the NSAIDs, besides their well established role in relieving pain, fever and inflammation, have shown an emerging potential for the chemoprevention of cancer in colon and other tissues.2, 3 There are two COX isoforms; COX-1 and COX-2 with distinct tissue distributions and physiological functions.4 COX-1 is constitutively expressed in many tissues, whereas the inducible isoenzyme COX-2 is proinflammatory in nature and expressed only in response to certain stimuli such as mitogens, cytokines and growth-factors. 5, 6 The etoricoxib, an important new member of this class of drugs called the selective COX-2 inhibitors or coxibs, which had a bipyridine structure, showed favorable tissue retention and particularly suitable for the long term cancer chemopreventive effects because of the lack of its known side effects.7
Previous studies from this laboratory have shown that COX-2 is upregulated in experimental colon carcinoma and its inhibition is associated with a reduction in tumorigenesis by etoricoxib.8, 9 However, the mechanism responsible for the antitumor effects of such COX-2 inhibitors had not been clearly defined. The present paper showed that apoptosis is the dominant anti-proliferative end effect of this potentially effective chemopreventive compound.
Materials and methods
Animals
Six to eight week-old, 80-100 g body weight of male Sprague-Dawely rats were obtained from the Central Animal House of Panjab University, Chandigarh. The animals were housed in polypropylene cages, embedded with rice husk and maintained under hygienic conditions on standard animal feed and a free access to water. The body weights of the rats were recorded every week. All of the animal procedures reported here are according to the guidelines approved by the Ethical Committee of Panjab University for the use of experimental animals for biomedical research.
Experimental design
Rats were randomly assorted and bodily marked for identification. Forty rats were divided into the following experimental groups: group 1, Control (vehicle treated); group 2, DMH treated; group 3, DMH + Etoricoxib; Group 4, Etoricoxib treated. DMH was given weekly at the dose of 30 mg/kg body weight subcutaneously for 6 weeks. Etoricoxib was given daily at the rate of 0.6 mg/kg body weight orally. 1,2 dimethylhydrazine was obtained from Sigma Chemicals Co. (St. Louis, MO, USA) and prepared fresh every week in 1 mM EDTA saline, pH being adjusted to 7.0 using NaOH solution, immediately before subcutaneous injection. Etoricoxib was obtained from Ranbaxy Research Lab (Gurgaon, India) and prepared in the reported anti-inflammatory dose freshly in 0.5% sodium carboxymethyl cellulose. The body weights of all the rats were recorded weekly until the termination of the experiments.
Gross anatomical observation
All the animals were sacrificed in an overnight fasted condition, 6 weeks after the beginning of the study. They were sacrificed under an overdose of ether anesthesia and around 8 AM to avoid diurnal variation in the parameters studied. Colons were removed, flushed with physiological saline and opened longitudinally. They were thoroughly examined macroscopically for the presence of the multiple plaque lesions (MPLs), identifiable as the raised or non-raised mucosal plaques or nodular lesions on colonic mucosal surface and considered as the early centers of tumorigenesis.8 The chemopreventive response was assessed on the basis of MPL incidence, burden and multiplicity.
Staining and counting of aberrant crypt foci (ACF)
The colonic mucosa was spread flat on glass plate, fixed in 10% formalin PBS for overnight, stained with methylene blue (0.1% in PBS, pH 7.1) to aid visualization of the ACF.10 The colons were flooded with the stain and after 3-4 min the excess stain was rinsed off with PBS. The ACF were classified as small (1-3), medium (4-6), or large (> 6) by the number of crypts per foci.11, 12 The number and size of ACF in each colon were counted and recorded.
Histopathological observations
The colonic segments containing the MPL were dissected, fixed immediately in 10% formalin and paraffinembedded, sectioned, and stained with hematoxylin and eosin (H&E) for the histopathological observations.
Immunohistochemistry of COX-2 protein
About 7μm thick paraffin sections of rat colon were incubated at 60º C in an oven for 30 min for antigen retrieval and deparaffinized in xylene for 30 min. The sections were then gradually hydrated in a descending series of alcohol (100%, 90%, 70%, 50%, and 30%). The non-specific staining was blocked by incubating the sections with 2% BSA in phosphate buffered saline (PBS, 10mM, pH 7.2 Himedia, Mumbai, India). The sections were then incubated with the goat polyclonal antibody raised against rat COX-2 (1:2000 dilutions) (Santa Cruz, CA, USA) in a moist chamber for 2 hr at 37o C. For negative control only 1% BSA was used. After incubation with the primary antibody, washing was given with PBS, PBS tween (PBS with 0.05% tween 20) and PBS, successively, for 5 min each. The sections were then incubated with the alkaline phosphatase coupled secondary antibody for 2 h. Sections were washed again in the same manner as described above and the reaction product developed using BCIP/NBT (Genie, Bangalore, India) under dark conditions. Reaction was terminated by washing with distilled water, sections mounted with DPX and observed under a light microscope.
Isolation of colonocytes
Colonic epithelial cells (colonocytes) were obtained from the freshly isolated colons by the method of Mouille at al (2004),13 as originally described by Roediger and Truelove (1979).14 The colonic segments were removed and flushed with chilled physiological saline (NaCl solution, 9g/l) and then with a Ca2+- and Mg2+ - free Krebs-Hanseleit (KH) bicarbonate buffer (pH 7.4) containing 10 mM HEPES, 5mM dithiothreitol, and 2.5 g/l bovine serum albumin (BSA). The K-H buffer was equilibrated against a mixture of O2 and CO2 (19:1, vol/vol). Then, each colon was everted, distally ligated, and distended as much as possible by means of a syringe containing calcium free K-H saline with 0.25% w/v BSA. The proximal end of the colon was now ligated and placed in a plastic flask containing 100ml calcium-free K-H buffer, 5mMole/ l EDTA, and 0.25% BSA. The flask was gassed with O2+ CO2 (19:1 v/v) and incubated at 37o C in a shaking water bath at 60-70 oscillations per min for 30 min. Thereafter, the colons were rinsed in fresh calcium-free saline to remove the excess EDTA and placed in a plastic beaker with 50 ml K-H buffer with 0.25% w/v BSA. Manual stirring with a plastic stirrer for two min readily disaggregated the colonocytes, which were then separated by centrifugation at 500 g for 60 sec. The cells were washed two times in an oxygenated K-H buffer containing normal amounts of calcium (2.5 mMole/l CaCl2), 5 mMole/l DL-dithiothreitol (DTT), and 2.5% w/v BSA. The pellet was resuspended in 5 ml of the same saline by being taken up several times into a 10 ml polypropylene pipette.
The integrity of the cell membrane of the colonocytes was assessed by the ability of the cells to exclude vital dyes. Nuclear staining of trypan blue was used to detect the damaged cell membranes. Cells were permeable to trypan blue if nuclear staining occurred. The proportion of the permeable cells is expressed as a percentage of the total cell population counted in a hemocytometer chamber. The number of colonocytes resuspended in DMEM buffered with MOPS (pH 7.5, 25 mMole/l) were counted on a hemocytometer and it was determined that the isolation procedure used in these studies led to the recovery of the viable colonocytes, to the extent of at least 97% of the isolated cells.14
Apoptotic studies
Acridine orange-ethidium bromide co-staining
Acridine orange staining procedure was performed according to the method of Baker et al (1994).15 Briefly, the cells were suspended in PBS (pH 7.4) containing acridine orange (1 μg/ml) and RNaseA (Sigma, St. Louis, MO, USA) as well as the co-staining with ethidium bromide (Sigma, St. Louis, USA) in the same concentration. 16 Then the cells were washed and examined under fluorescence microscope (x 100) (Axioplan, Zeiss, Germany). For quantification of the apoptotic cells, a total of 100 cells from four different slides were observed and percentage of apoptotic cells calculated for the individual animal.
Hoechst 33342-propidium iodide co-staining
Hoechst 33342 dye is used to stain the DNA where the staining procedure was performed by the method of Yuan et al. (2002).17 Briefly, to 100 μl of the harvested cells, 2 μl of 1mg/ml propidium iodide (PI) (Sigma, St. Louis, USA) was added. The mixture was incubated at 37ºC for 10 min under dark conditions. After this, 2 μl of 1mg/ml Hoechst 33342 dye (Sigma, St. Louis, USA) was added. Cell suspension was kept at room temp for 5 min. 50 μl of the suspension was placed on a clean glass slide and examined under a fluorescence microscope. The percentage of apoptotic cells was calculated by counting 100 cells on separate slides as above.
Single cell gel electrophoresis (Comet assay)
Frosted slides were rinsed by methanol before use and air dried. A thin layer of regular melting agarose (RMA) was made; above it low melting agarose (LMA) mixed with the sample (colonocytes) in the ratio of 3:7 was placed followed by one more layer of RMA. The slides were then immersed in the lysis solution to allow the lysis of cells for 1 hr at 4o C, washed in distilled water and transferred in alkaline buffer for 20 min at 40C. After alkali unwinding of the DNA, these were electrophoresed in tris borate-EDTA buffer, pH 8.1. Electrophoresis conditions were 25 V and 300 mA for 20 min. After electrophoresis, the slides were immersed in the neutralizing buffer three times for 2-3 min, staining of the slides done by ethidium bromide for 20 min and observed under fluorescent microscope at 450x for comet assay using a 550-560 nm excitation and 590 nm barrier filter.18
Carrageenan induced inflammatory studies
Five groups of rats with four animals per group were treated as follows: Group 1: Control receiving 10 ml/kg saline, Group 2: receiving 0.32 mg/kg body weight of etoricoxib orally, Group 3: receiving 0.64 mg/kg body weight of etoricoxib, Group 4: receiving 1.28 mg/kg body weight of etoricoxib and Group 5: receiving indomethacin as reference at the rate of 5 mg/kg orally. The paw volume in the left hind leg of the animals was measured before the start of the experiment in a plethysmometer and also after that. After 1 hr, 0.1 ml of 1 % carragenan in saline was injected in the paw and the paw volume measured at 0, 1, 2, 3 and 4 hrs.19 The percent swelling caused by the drug was calculated by the formula:
(V-Vt/Vt) x 100
Where: V = mean oedema volume of the rats in the control group.
Vt = mean oedema volume of rats in the test group.
While percent inhibition was calculated by the formula:
1-% swelling of drug treated x 100/% swelling of control.
Results
The present study was designed to evaluate the chemopreventive response of COX-2 selective drug, etoricoxib in a chemically induced colon carcinogenesis. Figure 1 shows the body weight profile during the treatment schedule. All the groups showed normal body growth and gained almost equal weight when compared to their initial body weight (fig. 1).
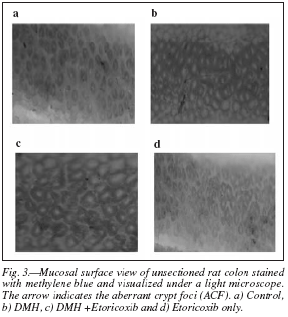
Figure 2 shows gross anatomy of the mucosal surface of colon depicting the occurrence of multiple plaque lesions (MPLs) of (a) Control, (b) DMH, (c) DMH + Etoricoxib and (d) Etoricoxib only. MPLs were observed in almost all the DMH treated rats (90%). DMH + Etoricoxib treated group had less MPL as compared to the DMH group (40%) and minimum number of MPL noted in the etoricoxib only group (20%). No MPL was identified in the control animals (table I).
The aberrant crypt foci (ACFs) were identified as the discrete aggregation of aberrant/abnormal crypts which are widened and localized among the normal appearing rest of the crypts and staining differently (fig. 3). The ACF were counted in equal sized areas and also from equal proportion of the colon. No ACF were found in the control animals while the DMH group has a large number of ACF (33) with 13 small, 11 medium and 9 large ACF. ACFs were classified as small (1-3), medium (4-6) or large (> 6) by the number of crypts per foci (table II).
Histopathologic analysis in the control rats of the colon revealed no signs of adenocarcinoma and reported the normal epithelium (fig. 4). DMH group reported the presence of hyperplastic epithelium particularly in the proximal region. The crypts were found to be enlarged along with the distinct inflammatory changes. Dysplastic crypts with apparent neoplastic changes are also recorded in these animals. DMH + Etoricoxib group reported occasional occurrence of the hyperplasia while none of these were found in Etoricoxib alone group.
Immunohistochemical analysis of COX-2 protein (fig. 5) in the colonic segments of the different groups revealed the absence of COX-2 protein in the Control, while section from the DMH treated animals shows the intense cytoplasmic expression of COX-2 in the various locations of the mucosal surface. DMH + Etoricoxib showed very low level of COX-2 protein while Etoricoxib only group had a minimum expression of it.
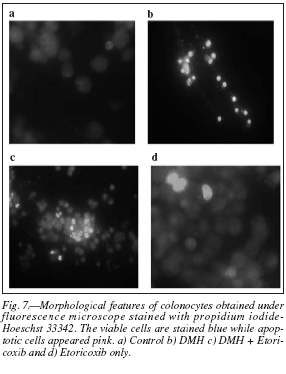
Apoptosis was studied by the DNA binding dyes acridine orange-ethidium bromide and Hoechst 33342-propidium iodide co-staining. Apoptotic cells were indicated as brightly labeled nuclei (orange colored with acridine orange-ethidium bromide and pink with Hoechst 33342-propidium iodide). Figure 6 showed the photomicrographs of normal, apoptotic and necrotic (dead) cells as observed in the various treatment groups in acridine orange-ethidium bromide costaining while the propidium iodide-Hoechst 33342 co-staining was depicted in the figure 7. The number of apoptotic cells were counted in a fluorescent microscope and presented in Figure 8 which showed that only a minimum number of apoptotic cells were present in the DMH group while an increased number of these cells observed in DMH + Etoricoxib group. Etoricoxib only group showed a few apoptotic cells, and in general comparable to the Control group.
Further, the DNA damage caused in the cell as a result of DMH was studied by the single cell gel electrophoresis (comet assay) (fig. 9). Results indicate that the DNA of the DMH treatment groups was clearly damaged as evident by the comet formation with elliptical shape in the form of a tail as compared to the normal DNA, which was found to be reversed due to Etoricoxib treatment. The DNA damage of the affected cells in percentage terms was found to be 60% in the DMH group as compared to the 5% cases in the Control while in DMH + Etoricoxib group the DNA damage was found to be 29%. The etoricoxib only group showed 8% DNA damage with almost circular shaped DNA.
It was also important to study the anti-inflammatory property of Etoricoxib, in particular at a dose similar to the one used in the present anti cancer study. Therefore with etoricoxib also, carrageenan-induced paw oedema was measured, which shows the complete inhibition of oedema (fig. 10). The percent inhibition in the oedema formation was depicted in table III which shows that at a dose 0.64 mg/kg body weight, the percent swelling at 4 hrs of the drug treatment was reduced to 3.2% from an early value of 17.24 (without any drug) which amounted to an inhibition of about 81%. The reference drug, indomethacin also produced the identical amount of reduction in swelling (3.2%) of the carrageenan induced rat paw edema formation which also amounted to an identical inhibition of 81%.
Discussion
Recent studies have shown the potential use of selective COX-2 inhibitors in the treatment and prevention of colon cancer.20 Growth inhibition and apoptosis have also been observed in some cancer lines by COX-2 inhibitors.21 However, there are very few studies of such chemopreventive action of colon cancer in animal models with selective COX-2 inhibitors and their molecular mechanism established. Therefore, the present study was undertaken to test the effect of etoricoxib, a recently introduced COX-2 inhibitors with a bipyridine nucleus as the chemopreventive response in rat in the initiation stage (6 week treatment) of colon carcinogenesis induced by DMH a known procarcinogen which specifically affects the colon.11 In this study we have analyzed the regression of the preneoplastic lesions, histopathological evaluation of the prognostic markers the inhibition of the COX-2 expression and the antiproliferative and proapoptotic effects of etoricoxib. These studies demonstrate that premalignant signs such as the occurrence of multiple plaque lesions and ACF (both hyperplastic and dysplastic type) along with aggregates of lymphoid follicles and dysplasia were clearly visible in the DMH treated rats which were found to be greatly regressed in the etoricoxib treatment. Various studies involving DMHinduced colon carcinogenesis in rat model indicate that morphological surface alterations such as the proliferative activity and dysplasia in colonic mucosal epithelium start developing during the initiation stage of carcinogenesis itself20 and the dysplastic ACFs overlaying the lymphoid aggregates are considered as the earliest identified putative premalignant precursors of both human and experimental colon cancer.22
The results of the present study thus clearly demonstrate that regular administration of a specific COX-2 inhibitor at its anti-inflammatory dose brings about lesser inflammation; lesser dysplasia and reduced ACF associated aggregates of lymphoid follicles indicating the chemopreventive effects during an early stage of colon carcinogenesis. Although the molecular mechanism to such reduction in colon carcinogenesis is unknown, a likely possibility is through the blockage of COX enzyme, which in turn suppresses the eicosanoid production and specially the type 2 series of prostaglandins that affect the cell proliferation, tumor growth and immune responsiveness.23 Results of the present study clearly demonstrate the much reduced cytoplasmic presence of COX-2 with etoricoxib treatment which is otherwise highly expressed in the DMH group. COX-2 isoform of the cyclooxygenase enzyme is known to be an early response inducible gene product against many agents such as the genotoxic compounds, mitogens, cytotoxins, growth factors and also expressed heavily in colon and other forms of cancer.24
Apoptosis or programmed cell death is the central element in the pathogenesis of many disease processes and in the response to systemic therapies in the neoplastic cells.25,26 Caspases have been identified as the effective agents of such apoptotic process, and DNA fragmentation as well as nuclear morphological changes has been placed downstream of the caspase activity.27 Cells undergoing apoptosis exhibit specific morphological changes, which include membrane blebbing, cytoplasmic and chromatin condensation, nuclear breakdown and formation of apoptotic bodies, and the affected cells are eventually subjected to phagocytosis.28 The present study clearly demonstrates an increased number of apoptotic cells in the DMH + Etoricoxib treated group as opposed to the DMH alone as evident by the apoptotic specific fluorescent staining.
The number of cells having DNA damage was also evaluated by a single cell gel electrophoresis method (Comet assay) which shows that a very high number of cells from the DMH treated groups had undergone DNA damage which had once again been highly restored in the presence of Etoricoxib. The DNA damage by comet assay is also a useful indication in the cancer induction and its chemoprevention studies.18
Comet assay is a method of measuring DNA damage to the individual cells based on the technique of microelectrophoresis where cells embedded in agarose and lysed, subjected briefly to an electric field, stained with a fluorescent DNA-binding stain and viewed using a fluorescence microscope. Broken DNA strands migrate in the electric field and resemble comet with a brightly focused head and a conspicuous tail region which increases as the DNA damage increases. DMH treatment has been shown to induce significant increase in the leukocyte DNA damage.29 Therefore, DNA damage has been implicated as the initial step in chemical carcinogenesis, while the blocking of DNA damage should constitute the first line of defense against cancer induction (Barth et al, 2005).30
It was also important to note that the specific COX-2 inhibitor used for the anticancer study should be within the anti-inflammatory dose range.31 This was evaluated through a carrageenan induced hind leg paw oedema studies where the Etoricoxib was found to be highly effective as an anti-inflammatory agent within the dose range which have also been used in the present study for the anti-cancer effect. Carrageenan test was selected because of its sensitivity in detecting orally active anti-inflammatory agents, particularly in the acute phase of inflammation.32, 33
While the ultimate goal of all anticancer strategies is to eliminate tumor cells, induction of apoptosis is by far the most pharmacologically preferred form of cell growth inhibition. A rapid death of cancer cells is not only needed to achieve tumor regression but also to reduce the risks of selecting more aggressive and drug resistant phenotypes that are responsible for tumor growth and treatment failure.34 In conclusion we present evidence that the etoricoxib, a bipyridine molecule and selective COX-2 inhibitor induces apoptosis in experimental colon carcinogenesis and therefore it could be a potential candidate for developing a new anticancer drug for the treatment of colon cancer and other cancers of epithelial origin.
Referencias
1. Smith WI, Meade EA, Dewitt DL. Interaction of PGH synthase isozyme-1 and 2 with the NSAIDs. Ann NY Acad Sci 1994; 744: 50-57. [ Links ]
2. Marnett LJ. Aspirin and the potential role of prostaglandin in colon cancer. Cancer Res 1992; 52: 5575-5589. [ Links ]
3. Duperon C, Castongnoy A. Chemopreventive efficiencies of aspirin and sulindac against lung tumorigenesis in A/J mice. Carcinogenesis 1997; 18: 1001-1006. [ Links ]
4. Smith NT, Langenbach R. Why are there two cyclooxygenase enzymes? J Clin Invest 2001; 167: 1491-1495. [ Links ]
5. Levy GN. Prostaglandin H synthase, non-steroidal anti-inflammatory drugs and colon cancer. FASEB J 1997; 11: 234-247. [ Links ]
6. Smalley WE, DuBois RN. Colorectal cancer and non-steroidal anti-inflammatory drugs. FEBS Left 1997; 11: 234-247. [ Links ]
7. Warner TD, Mitchell JA. Cyclooxygenase: new forms, new inhibitors and lessons from the clinic. FASEB J 2004; 18: 790-804. [ Links ]
8. Kanwar SS, Vaiphei K, Nehru B et al. Chemopreventive effects of non-steroidal anti-inflammatory drugs in the membrane lipid composition and fluidity parameters of the 1,2 dimethyl hydrazine induced colon carcinogenesis in rats. Drug Chem Toxicol 2007; 30: 293-309. [ Links ]
9. Kanwar SS, Vaiphei K, Nehru B et al. Antioxidative effects of non steroidal anti-inflammatory drugs during the initiation stages of experimental colon carcinogenesis in rats. J Envir Pathol Tox Oncol 2008; 27(2): 89-100. [ Links ]
10. Bird RJ. Observation and generation of aberrant crypts in the murine colon treated with a colon carcinogenesis: Preliminary findings. Cancer Lett 1987; 37: 147-151. [ Links ]
11. Park H-S, Goodlad RA, Wright NA. The incidence of aberrant crypt foci and colonic carcinoma in dimethyl hydrazine treated rats varies in a site -specific manner and depends on tumor histology. Cancer Research 1997; 57: 4507-4510. [ Links ]
12. Paulson JE, Lqberg EM, Qlstora HB et al. Flat dysplastic aberrant crypt foci are related to tumorigenesis in the colon of azoxymethane-treated rats. Cancer Res 2005; 65(1): 121-129. [ Links ]
13. Mouille B, Robert V, Blachier, F. Adaptive increase of ornithine production and decrease of ammonia metabolism in rat colonocytes and hyperproteic diet ingestion. Am J Physiol 2004; 287: G344-G351. [ Links ]
14. Roediger WEW, Truelove SC. Method of preparing isolated colonic epithelia cells (colonocytes) for metabolic studies. Gut 1979; 20: 484-488. [ Links ]
15. Baker AJ, Mooney A, Hughes J et al. Mesengial cell apoptosis: The major mechanism for resolution of glomerular hypercellularity in experimental mesengial proliferative nephritis. J Clin Invest 1994; 94: 2105-2116. [ Links ]
16. Schwartz LM, Obsorne BA. Cell death. Method Cell Biol 1995; 46: 15-18. [ Links ]
17. Yuan Y, Zhi-Qiang GE, Jing-Chuan L. Differentiation of apoptotic and necrotic cells in suspension cultures of Taxus cuspidate by the combined use of fluorescent dying and histochemical staining methods. Biotechnol Lett 2002; 24: 71-76. [ Links ]
18. Singh NP, McCoy MT, Tice RR et al. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 1988; 175: 184-191. [ Links ]
19. Gupta GD, Gaud RS. Anti-inflammatory activity of tenoxicam gel on carrageenan induced paw oedema in rats. Indian J Pharm Sci 2006; 68(3): 356-359. [ Links ]
20. Shiptz B, Klein E, Bklan G et al. Suppressive effects of aspirin on aberrant crypt foci in patients with colorectal cancer. Gut 2003; 52: 1598-1601. [ Links ]
21. Subhashini J, Mahipal SVK, Reddanna P. Antiproliferative and apoptotic effects of celecoxib on human chronic myeloid leukemia in vitro. Cancer lett 2005; 224: 31-43. [ Links ]
22. Wargovich MJ, Jimenez A, McKee K et al. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis 2000; 21(6): 1149-1155. [ Links ]
23. Sheng H, Shao J, Morrow JD et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998; 58: 362-366. [ Links ]
24. Dannenberg AJ, Altork NK, Boyle JC et al. Cyclooxygenase-2: a pharmacological target for the prevention of cancer. Lancet Oncol 2001; 2: 544-551. [ Links ]
25. Smets A. Programmed cell death (apoptosis) and response to anticancer drugs. Anticancer Drugs 1994; 5: 3-9. [ Links ]
26. Tsuji M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cell over expressing prostaglandin endoperoxide synthase 2. Cell 1995; 83: 493-501. [ Links ]
27. Fisher U, Jamicke RU, Schmlz-Osthof K. Many cuts to rain: a comprehensive uptake of caspase substrates. Cell Death Diff 2003; 10: 76-100. [ Links ]
28. Janicke R, Sprengart Ml, Wati MR et al. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998; 273: 9357-9360. [ Links ]
29. Park EJ, Kim KT, Kim CJ et al. Anticarcinogenic and Antigenotoxic Effects of Bacillus polyfermenticus. J Microbiol Biotechnol 2004; 14(4): 852-858. [ Links ]
30. Barth SW, Fahndrich C, Bub H et al. Cloudy apple juice decreases DNA damage, hyperproliferation and aberrant crypt foci development in the distal colon of DMH-initiated rats. Carcinogenesis 2005; 24: 1414-1421. [ Links ]
31. Blobaum AL, Marnett LJ. Molecular determinants for the selective inhibition of cyclooxygenase-2 by lumiracoxib. J Biol Chem 2007; 282 (22): 16379-16390. [ Links ]
32. Owoyele VB, Wurada CO, Soladoye AO et al. Studies on the anti-inflammatory and analgesic properties of Tilhonic diverfolia leaf extract. J Ethnopharmacol 2004; 90: 317-321. [ Links ]
33. Sharma JN, Samuel AM, Asmawi MZ. Comparison between plethysmometer and micrometer method to measure acute paw oedema for screening anti-inflammatory activity in mice. Inflammaopharmacol 2004; 12 (1): 89-94. [ Links ]
34. Roy KR, Arunasree KM, Dhoot A et al. C-phycocyanin inhibits 2-acetylaminofluorescence induced expression of MDR-1 in mouse macrophages cells: ROS mediated pathway determined via combination of experimental and in silico analysis. Arch Biochem Biophys 2007; 459: 169-177. [ Links ]
![]() Correspondence:
Correspondence:
S. N. Sanyal.
Professor, Department of Biophysics.
Panjab University, Chandigarh.
India-160014.
E-mail: sanyalpu@gmail.com
Recibido: 15-X-2009.
Aceptado: 11-XI-2009.