My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.25 n.2 Madrid Mar./Apr. 2010
Major diet-drug interactions affecting the kinetic characteristics and hypolipidaemic properties of statins
Principales interacciones dieta-fármaco que afectan la cinética y propiedadas hipolipemiantes de las estatinas
M. P. Vaquero1, F. J. Sánchez Muniz2, S. Jiménez Redondo2, P. Prats Oliván2, F. J. Higueras2 and S. Bastida2
1Departamento de Metabolismo y Nutrición. Instituto del Frío. Instituto de Ciencia y Tecnología de Alimentos y Nutrición (ICTAN). Consejo Superior de Investigaciones Científicas (CSIC). Madrid. 2Departamento de Nutrición y Bromatología I (Nutrición). Facultad de Farmacia. Universidad Complutense de Madrid. España.
The study was partially granted by Projects PR248/01-10161 and AGL 2008-04892-C03-02.
ABSTRACT
Concomitant administration of statins with food may alter statin pharmacokinetics or pharmacodynamics, increasing the risk of adverse reactions such as myopathy or rhabdomyolysis or reducing their pharmacological action. This paper reviews major interactions between statins and dietary compounds. Consumption of pectin or oat bran together with Lovastatin reduces absorption of the drug, while alcohol intake does not appear to affect the efficacy and safety of Fluvastatin treatment. Grapefruit juice components inhibit cytochrome P-4503A4, reducing the presystemic metabolism of drugs such as Simvastatin, Lovastatin and Atorvastatin. Follow-up studies on the therapeutic effect of statins in patients consuming a Mediterranean-style diet are necessary to assure the correct prescription because the oil-statin and minor oil compound-statin possible interactions have been only briefly studied. Preliminary study suggests that olive oil can increase the hypolipaemiant effect of Simvastatin with respect sunflower oil. The consumption of polyunsaturated rich oils, throughout the cytochrome P- 450 activation could decrease the half-life of some statins and therefore their hypolipaemic effects. The statins and n-3 fatty acids combined therapy gives rise to pharmacodinamic interaction that improves the lipid profile and leads greater cardioprotection. Although statins are more effective in high endogenous cholesterol production subjects and plant sterols are more effective in high cholesterol absorption efficacy subjects, plant esterols-statins combined therapy generates very positive complementary effects. This review ends suggesting possible diet-stain interactions that require further investigations (e.g. types of olive oils, fruit juices other than grapefruit, fibre or consumption of alcoholic beverages rich in polyphenols or ethanol).
Key words: Statins. Diet-drug interactions. Fibre. Alcohol. Grapefruit juice. Oils. Plant sterols. N-3 fatty acids.
RESUMEN
La administración conjunta de estatinas y alimentos puede producir reacciones adversas, como miopatía o rabdomiolisis, o reducir su acción farmacológica. Este artículo revisa las interacciones más importantes entre estatinas y componentes dietéticos. El consumo de pectina o de salvado de avena junto con Lovastatina reduce la absorción del fármaco, mientras que la ingesta de alcohol no afecta la eficacia ni la seguridad del tratamiento con Fluvastatina. Algunos componentes del zumo de pomelo inhiben el citocromo P-450 A4, reduciendo el metabolismo presistémico de la Simvastatina, Lovastatina y Atorvastatina. Se necesitan estudios de seguimiento sobre los efectos terapéuticos de las estatinas en pacientes que consumen dieta Mediterránea para asegurar la correcta prescripción, ya que no existen estudios de posibles interacciones entre el aceite o sus compuestos minoritarios y las estatinas. Un estudio preliminar sugiere que el aceite de oliva respecto al de girasol incrementa la acción hipolipemiante de la Simvastatina. El consumo de aceites ricos en ácidos grasos poliinsaturados, a través de la activación de citocromo P-450, podría disminuir la vida media de las estatinas y, por tanto, sus efectos hipolipemiantes. La terapia combinada estatinas y ácidos grasos n-3 da lugar a una interacción farmacodinámica que mejora el perfil lipídico e induce mayor cardioprotección. Aunque las estatinas son más efectivas en individuos con alta producción endógena de colesterol y los esteroles de plantas en aquellos que presentan mayor absorción de colesterol, la terapia conjunta induce efectos complementarios muy positivos. Se concluye la revisión sugiriendo más estudios en aquellas interacciones entre alimentos (ej. tipos de aceite de oliva, de zumos, de fibra o el consumo de bebidas alcohólicas ricas en polifenoles o etanol) y estatinas.
Palabras clave: Estatinas. Interacciones dieta-fármacos. Fibra. Alcohol. Zumo de pomelo. Aceites. Fitosteroles. Ácidos grasos n-3.
Introduction
Statins are drugs that inhibit the rate-limiting enzyme of cholesterol synthesis 3-hydroxy-3-methylglutaryl coenzyme A reductase, thus reducing intracellular hepatic cholesterol biosynthesis and decreasing intracellular cholesterol accumulation. The reduction in intracellular cholesterol levels stimulates synthesis of low density lipoproteins (LDL) receptors and their expression on the surface of liver cells. These receptors are responsible for the uptake of LDL in addition to that of their precursors, very low density lipoproteins (VLDL) and VLDL remnants, whose hydrolysis produces LDL. The more VLDL and VLDL remnants taken up by the receptors, the fewer LDL produced. This effect of statins on VLDL also explains their capacity to reduce triglyceride levels, although to a lesser degree and in a less consistent manner.1 Nevertheless, statins may also produce adverse reactions, such as myopathy and rhabdomyolysis. Concomitant use of other drugs may lead to undesirable interactions and increase the risk of adverse reactions.2 Of the various statins available, those that are presently marketed in Spain include Simvastatin, Lovastatin, Pravastatin, Atorvastatin and Fluvastatin (fig. 1).
The knowledge of structure and pharmacokinetic characteristics of these drugs will help to understand their pharmacokinetically-based interactions with certain food compounds. Table I summarises the pharmacokinetic characteristics of statins, as well as the principal transporters involved in their metabolism and elimination. All statins presently available display good intestinal absorption. Thus, Simvastatin and Lovastatin, which are lipophylic, easily cross the plasma membrane by means of passive diffusion, while other statins are recognised by specific transport systems. Pravastatin is the least lipophylic statin.2,3 Simvastatin, Lovastatin, Pravastatin, Atorvastatin and Pitavastatin are P-glycoprotein (Pgp) substrates. The Pgp is a membrane glycoprotein which plays an important role during the cellular capture of drugs.3
The statins presently available are mainly eliminated by the liver. Except for Pravastatin, of which up to 47% is eliminated through urine in an unaltered form, urinary excretion of statins is limited (table I). Organic anion transporter OAT3 may be involved in renal Pravastatin uptake.3
Simvastatin, Lovastatin and Atorvastatin are metabolised by cytochrome P-450 3 A4 (CYP 3 A4), while Fluvastatin is metabolised by CYP 2 C9. Only small amounts of Pravastatin, Rosuvastatin and Pitavastatin are metabolised; their plasma clearance depends on transporters involved in hepatic uptake and bile excretion. Hepatic uptake by transporters also plays an important role in clearance of other statins administered in acid form (Fluvastatin and Atorvastatin).3
A pharmacological interaction is defined as an alteration of the pharmacodynamics and/or pharmacokinetics of a drug, produced by concomitant pharmacological treatment, dietary factors or social habits such as tobacco or alcohol.4 Such interactions can affect statins as much as any other drugs resulting in higher risk of adverse reactions in some cases or lower pharmacological action in others. This review summarises major data available on nutritional interactions with statins, placing special emphasis on those interactions that produce an additive effect to statin treatment.
Statins and dietary fibre
Although the effects of fibre on cholesterol levels have been thoroughly investigated and its benefits proven, 5 studies have shown that its consumption together with Lovastatin reduces intestinal absorption of the drug. In one study6 it was found that after addition of pectin (three patients) or oat/bran (two patients) to Lovastatin (80 mg/day), the LDL-c levels were increased after 4 weeks and returned to their previous values after the consumption of fibre was stopped. Later, it was observed that the combination of Lovastatin with psyllium lowered LDL-c more than the statin alone. Agrawal et al.7, in a 4-week parallel study involving 36 male adults, in which subjects consumed 20 mg of Lovastatin, 10 g of psyllium or 20 mg Lovastatin plus 10 mg psyllium, observed an additive effect of the combination, although the differences were not significant. In a 12- week blinded placebo-controlled study, patients were randomized to receive 20 mg of Simvastatin plus placebo, 10 mg of Simvastatin plus placebo, or 10 mg of Simvastatin plus 15 g of psyllium daily.8 The effect on lipid profile was evaluated at 4 and 8 weeks. It was concluded that dietary psyllium supplementation in patients taking 10 mg Simvastatin is as effective in lowering LDL-c and ApoB as 20 mg of Simvastatin alone. Values of HDL-c and TG did not significantly vary by the fibre addition. In this context, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend increase soluble fibre (10-25 g daily) as a therapeutic option to enhance LDL-c lowering.9 Viscous fibre may have additional side effects reducing blood glucose. Besides, it is important to take into account the dietary fibre, because administration of Lovastatin with food is recommended as under those conditions plasma concentrations are 50% higher than at fasting conditions.10.
Statins and alcoholic beverages
Moderate consumption of alcohol is known to have beneficial effects on cardiovascular disease, probably by increasing HDL-c levels. However, high consumption displays deleterious effects giving rise to hypertriglyceridaemia.11
Few studies have been carried out to determine the possible interactions between alcohol and statins (table II). A prospective crossover study investigated the effect of acute alcohol consumption together with a single 40 mg oral dose of Fluvastatin.12 After being randomly divided into two groups, individuals in one group were given a total of 70 g of ethanol in separate doses diluted to 20% with orange juice, while those of the other group were given orange juice alone. Volunteers given alcohol showed that the half-life (t1/2) of the single dose of Fluvastatin was significantly shorter than that of the controls, whereas the area under the curve (AUC), the maximum concentration (Cmax), and the time to reach maximum concentration (Tmax) did not differ from the control group. The alterations in the lipoprotein profile, measured after 8 hours, were observed when the statin was taken with alcohol. Triglyceride levels increased, total cholesterol and LDL-c levels decreased, possibly due to a pharmacokinetic interaction, and HDL-c and Apo A1 values slightly decreased.12 In contrast, the lipoprotein profile measured after 8 hours in the control group did not vary and only a slight reduction in Apo A1 was observed.
To evaluate the long-term effect of alcohol consumption, a prospective, double-blind crossed study involving 26 individuals with primary hypercholesterolaemia and a control group was carried out.13 Patients receiving Fluvastatin (40 mg/day) for 6 weeks were divided at random into two groups; one was given 20 g of alcohol diluted to 20% with lemonade while the other was given lemonade alone. Alcohol intake tended to increase the half life (t1/2), the area under the curve (AUC) and the time to reach maximum concentration (Tmax) compared with control values, while it did not appear to affect maximum concentration values (Cmax). With regard to the lipoprotein profile, both groups displayed a decrease in total cholesterol, LDL-c and Apo B, and no significant differences between the decreases in the two groups were observed, while HDL-c and triglyceride levels displayed no significant changes. Although alcohol modifies the metabolism of the drug, it does not reduce its efficacy as a hypolipidaemic agent.13
In conclusion, alcohol consumption does not affect the efficacy and safety of Fluvastatin treatment, although the effect of large doses of alcohol over long periods of time requires further investigation. Moreover, the concomitant effect of minor compounds present on alcoholic beverages (e.g. trans-resveratrol in red wines) on statin pharmacokynetics have not yet been investigated.
Statins and grapefruit juice
The first article to report an interaction between grapefruit juice and a drug appeared in 1989. This accidental finding, made by Bailey et al.14 while studying the haemodynamic effect of felodipine and ethanol, was due to the use of grapefruit juice to disguise the flavour of ethanol.
Grapefruit juice interacts with various drugs,15 including calcium channel blockers, antihistamines, immunosuppressants, hypnotic benzodiazepines, and the HMGCoA reductase inhibitors statins. The interaction mechanism is believed to be the irreversible inhibition that grapefruit juice produces on the intestinal cytochrome P-450 (CYP) 3A4, leading to a reduction of the presystemic metabolism of the drug, elevating its bioavailability. Alternative mechanisms of this interaction and the possible responsible compounds in the grapefruit juice are presented with more detail at the end of this section.
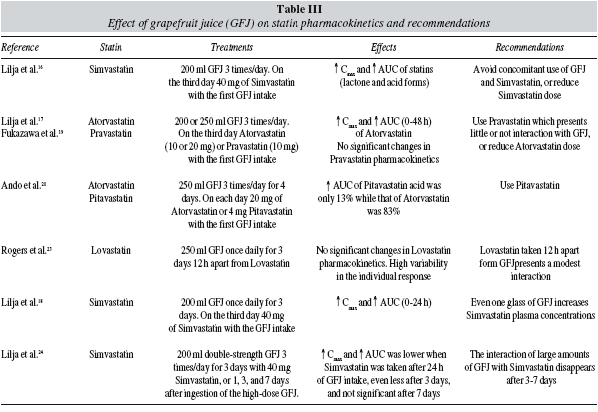
The pharmacokinetic effects of grapefruit juice on the various statins have been investigated in different studies carried out by Lilja et al.16-18 (table III). In one two-phased crossover study,16 10 healthy volunteers, divided at random into two groups, drank 200 ml grapefruit juice or water three times a day for two days. On the third day, each volunteer took 40 mg of Simvastatin with 200 ml of grapefruit juice or water and another 200 ml of the same liquid 30 minutes and 90 minutes after Simvastatin intake. The results of this study show an increase in the Cmax and the AUC of Simvastatin in volunteers who drank grapefruit juice. Cmax values and AUC also increased with the acid form of Simvastatin. The authors conclude that grapefruit juice increases serum concentrations of both, the lactone and acid form of Simvastatin, and suggest that the probable mechanism of action is the reduction of the presystemic metabolism of Simvastatin due to the inhibition of CYP 3A4. These findings indicate that concomitant administration of grapefruit juice and Simvastatin, at least in large quantities of the juice, should be avoided, or that the dose of Simvastatin should be reduced to avoid adverse reactions such as rhabdomyolysis.16
In a later study, the same research group17 studied the effect of grapefruit juice on the pharmacokinetics of Atorvastatin and Pravastatin, using similar design as in the previous study. Grapefruit juice significantly increases serum concentrations of Atorvastatin, in both its acid and lactone forms. Thus, concomitant administration of Atorvastatin and grapefruit juice should be avoided, or the dose of Atorvastatin reduced proportionately.16 Nevertheless, this juice does not appear to have any effect on the pharmacokinetics of Pravastatin due to its hydrosolubility. A Japanese study also confirmed the interaction of grapefruit juice with Atorvastatin but not with Pravastatin.19 It has been also found that Pitavastatin, unlike Atorvastatin, appears to be scarcely affected by the CYP 3A4-mediated metabolism.20
However, there is a debate concerning duration of the inhibitory effect of grapefruit juice on CYP 3A4 and the real effect in hypercholesterolaemic patients. It should be considered, on one hand the amount of grapefruit juice, its concentration and frequency of consumption, and on the other that the variability of the individual response to the statin-fruit juice interaction is very high,21-22 which may be explained by genetic variability.
Repeated consumption of high quantities of grapefruit juice has increased the AUC values of Simvastatin considerably.16 However, drinking daily 250 ml of the juice 12 h apart from Lovastatin has a modest effect on the pharmacokinetic of the drug.23
In a crossover study using 40 mg of Simvastatin with water (control period) and with high doses of grapefruit juice (200 ml three times a day for three days), or 1, 3 and 7 days after drinking the large quantities of grapefruit juice24 it was observed that when Simvastatin was taken with grapefruit juice, Simvastatin Cmax and AUC increased 12 and 13.5-fold, respectively, in comparison with control values. When Simvastatin was administered 24 hr after the last grapefruit juice intake, Cmax and AUC increased 2.4- and 2.1-fold, respectively, compared with control values. When Simvastatin was given 3 days after the grapefruit juice, Cmax and AUC increased 1.5- and 1.4-fold, respectively, in comparison with control values. There were no differences between Cmax and AUC values of study participants who took Simvastatin 7 days after taking grapefruit juice and controls. Furthermore, when Simvastatin was taken 24 hours after large amounts of grapefruit juice the AUC is much lower that the one observed after a concomitant intake of both. The potential interaction of large amounts of grapefruit juice with CYP 3A4 disappears 3-7 days after the last intake of grapefruit juice.24
Given that Simvastatin, Lovastatin and Atorvastatin are metabolised by CYP 3A4 (table I), they are the only available statins affected by this particular interaction, for which reason one of the other statins (e.g. Pravastatin, Pitavastatin) can be taken as an alternative treatment.
However, a number of studies focus on other interaction mechanisms besides that of CYP 3A4. Grapefruit juice also inhibits P-glycoprotein, a transporter that carries drugs from the enterocyte back to the gut lumen, resulting in a further increase in the fraction of drug that is absorbed.16,25 Grapefruit juice is also capable to inhibit human organic anion-transporting polypeptide B (OATP-B) in vitro.25-26 This inhibition decreases the intestinal uptake and therefore the oral bioavailability of the drug, which is the opposite effect to that of CYP 3A4 inhibition. Nevertheless, it remains unclear the contribution to the bioavailability of statins affected by P-glycoprotein and OATP-B inhibition.
In 2007, Li et al.27-28 described an estearase inhibition attribute of grapefruit juice as a new drug interaction. Lovastatin (a lactone) is known to be metabolized by CYP 3A4 but also by estearase to a hydroxyacid analog (active drug). The hydrolysis occurs in gut, liver and plasma and is considered its major metabolic pathway. The study carried out in rats and Caco-2 cells demonstrated that the grapefruit juice decreased Lovastatin hydrolysis in the gut, and thereby markedly increased the metabolic stability and permeability of the ester, leading to the enhancement of exposure to Lovastatin acid in rats. It was also found that the contribution of estearase inhibition was similar to that of CYP 3A, and that the estearase inhibition also affected Analapril (aprodrug for the treatment of hypertension) pharmacokinetics.
The potential interaction between specific grapefruit juice components has been also investigated.28-29 In one study, it was observed that the most powerful inhibitor of P-glycoprotein was the furanocoumarin 6,7-epoxy-bergamottin, followed by 6,7-dihydroxybergamottin, while bergamottin did not display any inhibitory effect at concentrations of up to 10 μmol. Naringenin was 10 times more powerful than naringin. Concentrations of the flavonoids and furanocoumarins evaluated in this study were in the same range as those found in grapefruit juice. Therefore, the results obtained from this in vitro study suggest that the compounds present in grapefruit juice are able to inhibit P-glycoprotein modifying the bioavailability of drugs such as Simvastatin, Lovastatin, Pravastatin, Atorvastatin and Pitavastatin, which are substrates for that glycoprotein.3 Heating grapefruit juice was recently found to decrease its concentrations of bergamottin and dihydroxybergamottin, thereby decreasing its interactions.30 This finding could be very useful to those patients who wish to drink this juice but are taking one of the drugs that interact with it.
Concerning the juice components responsible of the estearase inhibition, the furanocumarins bergamottin, 6,7-dihydroxybergamottin, and bergapten, and the glycoside flavonoids naringin and hesperidin, did not present estearase inhibition at concentration found in grapefruit juice or higher.28 However, the flavonoid aglycones morin, galangin, kaemferol, quercetin, and naringenin showed appreciable estearase inhibition. It should be pointed out that these compounds are widely distributed among fruit juices.
There are few studies on the possible interactions between fruit juices other than grapefruit and statins. Citrus juices have been found to interact with statins through the OATP mechanism.31 Orange juice increases Pravastatin AUC, without affecting its excretion rate.31 This effect could be related to higher intestinal absorption of the drug, mediated by the orange juice, but the mechanism is not clear and further studies are necessary. Orange juice does not alter Simvastatin pharmacokinetics.32
One report highlights the possible interaction between Rosuvastatin and pomegranate juice causing rhabdomyolysis in one patient.33 The patient was treated for familiar hypercholesterolemia with Ezetimide 10 mg/day and Rosuvastatin 5 mg every other day for 17 months. Three weeks before urgent presentation, and after reading the consumer information on the antioxidant benefits of pomegranate juice, he began drinking 200 ml twice weekly. Although the precise influence of this juice was not studied since this was only a case report, this observation deserves further research, including the impact of the media on patient's choice. These data point out that health claims of food and ingredients should be thoroughly evaluated considering all aspects of the target population.
Statins and unsaturated fatty acids
Statins and unsaturated oils
Very little information exists on the possible relation between unsaturated oil intake and statin effects. This is surprising because culinary oils contribute with more than 50% of the consumed fat.11 It has been known for many years that diets rich in fat and cholesterol tend to raise serum cholesterol.34-36 Monounsaturated fatty acids (MUFA) are considered neutral because they do not raise or lower the plasma cholesterol. However, recent studies show that replacing saturated fatty acids (SFA) with either MUFA or polyunsaturated fatty acids (PUFA) lowers total and LDL-c.37,38 Mattson and Grundy35 found that oleic acid lowered plasma cholesterol as much as linoleic acid. According to Dietschy,39 unsaturated fatty acids (oleic acid more than linoleic acid) increase gene expression of LDL receptors, maintaining the amount and activity of these receptors high and thus decreasing the concentration of serum LDL, while palmitic acid in the liver maintains gene expression of LDL-receptors low and, thus, serum LDL concentrations high. However, it is generally accepted a more prominent hypocholesterolemic effect of n-6 PUFA (e.g. linoleic acid) than of MUFA (e.g. oleic acid).40,41 Nonetheless, oleic acid keeps HDL-c higher than linoleic acid does.40,41
Taking into consideration all these results, our research group42 carried out a observational follow-up study in 25 men aged 45 to 65 years presenting according to the ATPIII protocol a cardiovascular risk higher than 20%. Twelve of the volunteers used daily, as the only culinary fat, olive oil; while the other thirteen volunteers used sunflower oil. After hypercholesterolaemia diagnosis, the participants were given statins for the first time. It was confirmed that statins, with independence of the consumed culinary oil, significantly reduced serum lipid and lipoprotein levels after 6 months of treatment. No significant differences on cholesterol, LDL-c, HDL-c and triglycerides were found at the start and six months later between the two oil groups. TC/HDL-cholesterol and the ATPIII 10-year risk percent significantly decreased more in the olive oil group. TC and the TC/HDL-cholesterol and the LDL-cholesterol/HDL-cholesterol ratios and the ATPIII 10-year risk percent decreased significantly more in the olive oil-group after BMI, energy and alcohol intakes were adjusted. Figure 3 shows the differences found in volunteers consuming Simvastatin and olive oil or sunflower oil. This result is relevant taken into account the predictive power of future cardiovascular risk of the TC/HDL-cholesterol ratio.43
At present no clear explanation can be drown taken into consideration the theoretical higher hypocholesterolaemic effect of linoleic acid than the oleic acid.40,41 However, PUFA have been shown to activate CYP activity, while SFA exert lower cytochrome activation with MUFA exerting intermedium effect.44 Thus, we suggest that the half-life of statins could be reduced due to a cytochrome-activating effect when the drug is consumed by patients following a sunflower oil rich diet with respect to the same diet prepared with olive oil. Nonetheless, the possible potentiating effect of olive oil polyphenols on statin pharmakocynetic, due to an inhibition of CYP,44,45 should not be ruled out. In the Sánchez-Muniz et al preliminary paper,42 it was concluded that although Simvastatin was a very effective hypolipaemic drug, olive oil-diets in preference to sunflower oil-diets should be consumed in patients with high cardiovascular risk.
Statins and n-3 fatty acids
Humans cannot produce n-3 fatty acids, for which reason they are essential and must be obtained from the diet. Eicosapentaenoic acid (EPA) is the precursors of a group of eicosanoids, including prostaglandins (PGE3, PGD3), prostacyclins (PGI3), thromboxanes (TXA3) and leukotrienes (LTB5), with anti-inflammatory, antithrombotic, antiarrhythmic and vasodilating properties.47 Numerous studies have demonstrated the cardioprotective effect of n-3 fatty acids,48,51 throughout reduction of TXA2 formation,11 and the increased synthesis of TXA3 and PGI3.52,53 N-3 fatty acids decrease formation of serie 4-leukotrienes by monocytes and neutrophils, attenuating leukotriene-mediated chemotaxis and the adherence of neutrophils to endothelial cells. They also reduce the formation of platelet derived growth factor,54 decreasing the proliferation of endothelial cells in the process of atherosclerosis, lower plasma fibrinogen levels55 and arterial pressure in individuals with hypertension.56
Results of the ATTICA Study57 showed that fish consumption was inversely related to all the inflammatory markers analysed, especially protein C reactive and interleukine-6, followed by tumour necrosis factor-α and the white blood cell count.
The anti-arrhythmic properties of n-3 fatty acids may constitute another protective mechanism against cardiovascular disease.58 Fish intake reduced the risk of sudden cardiac death in some studies.48,49 Moreover, n-3 fatty acids are able to alter cardiac electrophysiology and, thus, behave as pro or anti-arrhythmic agents, depending on the mechanism of the arrhythmia.58 Statins also display anti-arrhythmic properties, as it is shown in different studies.59-61 While this action mechanism of statins are not yet entirely clear, it appears that they may produce these effects due to their anti-inflammatory62 or anti-oxidant properties.63
The cardiovascular protective role of statins can be explained by their capacity to inhibit HMGCo-A reductase and reduce LDL-c levels in blood. Moreover, HMGCo-A reductase inhibitors are also known to inhibit transendothelial migration of neutrophils, as well as to alter the chemotactic capacity of monocytes.64 In addition, statins can also prevent cardiovascular disease through other mechanisms. These drugs inhibit synthesis of mevalonic acid, a precursor of cholesterol and other metabolites such as isopentenyl adenosines (which form part of tRNA) and dolichols (used in glycoprotein and CoQ10 synthesis). Furthermore, mevalonic acid metabolites, such as farnesyl pyrophosphate and geranyl pyrophosphate, mediate the prenylation of some specific proteins that intervene in transduction processes of cellular differentiation and proliferation. Thus, statins may protect against atherosclerosis by modulating cell function set into motion by the inhibition of protein prenylation.2
Statins and n-3 fatty acids have different but complementary effects on the lipid profile. While statins fundamentally reduce concentrations of cholesterol,65-66 n-3 fatty acids reduce plasma triglyceride levels, especially in individuals with hypertriglyceridaemia.67 A recent review of studies in humans68 reported that 1.5 to 3.5 g/day of n-3 fatty acids reduce plasma triglyceride concentrations by 25-30%, and that the majority of studies found reductions in total cholesterol and increases in HDL-c while the results on LDL-c are not consistent. Therefore, the association of statins with n-3 fatty acids could be useful to treat dyslipaemias characterised by high cholesterol and triglyceride levels.
Administered together, statins and n-3 fatty acids have proven to be safe, effective and well tolerated in treatment of combined dyslipaemia (table IV). In a study undertaken by our group,69,70 individuals having chronic statin therapy who consumed a fish-rich diet presented a better lipoprotein profile than those who ate a meat-based diet. In addition, a fish-rich diet is known to increase insulin sensitivity without the adverse effects of supplements containing high doses of n-3 fatty acids.68
In 32 patients with combined dyslipaemia,71 the addition of n-3 fatty acids to Pravastatin produced lower triglyceride and Apo B levels than Pravastatin treatment alone, although the differences were not statistically significant. On the other hand, total cholesterol values were significantly lower when Pravastatin was used with fish oil than when fish oil was given alone. Another study concluded that Simvastatin and n-3 fatty acids have an additive effect and that their combined therapeutic use probably has beneficial clinical consequences.72 When n-3 fatty acids were combined with Simvastatin treatment, total cholesterol and triglyceride levels decreased, while HDL-c concentrations were not affected. Apo A and Apo B values did not change, but Apo E concentrations decreased significantly.
The combined Simvastatin and n-3 fatty acid treatment reduced serum concentrations of total cholesterol, triglycerides and Apo E, as reported in previous studies.72 During postprandial hyperlipaemia, which decreased with both treatments (although significantly more with the combined treatment), activation of coagulation factor VII was significantly lower, indicating that combined treatment may reduce the thrombotic potential associated with intake of high-fat meals in these patients.73 Similar studies were carried out in patients treated with Atorvastatin. Nordøy et al.74 in a parallel-group, double-blind, placebo-controlled study found that postprandial levels of activated factor VII decreased with Atorvastatin treatment and diminished even further by the addition of n-3 fatty acids, which lowered the fasting and postprandial levels of activated factor VII, and its coagulant activity as well. Prothrombin fragment 1+2 increased during postprandial hyperlipaemia, but to a lesser degree after combined treatment with Atorvastatin and n-3 fatty acids. Therefore, although patients with combined hyperlipaemia present an elevated risk of activation of the coagulation system, especially during postprandial hyperlipaemia, combined statin and n-3 fatty acid therapy can significantly reduce this activation.74 In another study, 59 patients with coronary heart disease who received Simvastatin (10-40 mg/day) were divided at random into two groups for a one-year period, during which time one group received n-3 fatty acids while the other was given placebo.75 The addition of n-3 fatty acids significantly lowered serum triglyceride levels by 28% in 12 weeks, and by 35% after 48 weeks of treatment. In addition, VLDL-c concentrations significantly decreased and non-HDL-c levels dropped by 18% after 18 weeks of treatment.75 Nordøy et al.76 also concluded that the addition of low doses of n-3 fatty acids to Atorvastatin treatment could improve the cardiovascular disease risk profile in patients with combined hyperlipaemia.
Results regarding the efficacy of combined treatment with statins and n-3 fatty acids indicate that it is a useful therapeutic alternative in patients with hypertriglyceridaemia when LDL levels have decreased and statin monotreatment cannot lower hypertriglyceridaemia. As Simvastatin is extensively metabolised by CYP 3A4, McKenney et al.77 designed a random and double-crossed study, with two 14-day-long treatment periods separated by a wash-out period, to determine the impact of administration of n-3 fatty acids on Simvastatin pharmacokinetics. Participants were randomly divided into groups that received 80 mg of Simvastatin together with a daily morning dose of n-3 fatty acids or alone. No statistically significant differences in pharmacokinetic parameters of Simvastatin or its metabolite β-OH-Simvastatin were observed when Simvastatin was administered with n-3 fatty acids, making it possible to conclude that repeated doses of n-3 fatty acids do not affect Simvastatin pharmacokinetic. Nevertheless, after the first dose of fatty acids, the AUC of Simvastatin increased slightly. The authors of this study concluded that concomitant treatment with n-3 fatty acids and Simvastatin did not significantly affect the pharmacokinetics of Simvastatin in the stationary state and was well tolerated in the population studied.77
Statins and phytosterols
Sterols are a group of components essential to the formation of animal and plant cell membranes. Phytosterols are plant sterols and are differentiated from cholesterol by their lateral chain (fig. 2). Stanols are saturated sterols that lack the double bond in position 5 of the B ring. The most abundant dietary phytosterols are β-sitosterol, campesterol and stigmasterol.78
After the discovery of free phytostanols and their application to reduce cholesterol levels, investigations of fat-soluble phytostanol esters began in 1991,79 and phytostanol-enriched margarine became available to the general public in 1995.80 A 1992 study demonstrated that a dose of 1.5-3 g/day of phytosterols/phytostanols reduced total plasma cholesterol by 8-17% and LDL-c by 9-19%, while HDL-c levels were not affected.81 A subsequent meta-analysis of 41 assays confirmed those findings and also demonstrated an additive effect when they were taken with cholesterol-reducing drugs.82 The most important possible negative effect of phytosterol intake may be the reduction of fat-soluble vitamin plasma levels,78,83 which can be avoided, at least partially, with a fruit and vegetable-rich diet.
Phytosterols impede cholesterol absorption while statins inhibit cholesterol synthesis. Both mechanisms work in a complementary manner to increase LDL receptor activity and LDL-c plasma clearance. Statins are most effective in individuals with high endogenous cholesterol production, while plant sterols act best in those with the most efficient cholesterol absorption.82 The Scandinavian Simvastatin Survival Study (4S) demonstrated that individuals with the greatest cholesterol absorption efficiency and low cholesterol synthesis levels presented high blood phytosterol concentrations and were not able to reduce LDL-c levels or cardiovascular disease rates after 5 years of statin treatment. 84 Keeping in mind the effects of both, phytosterols and statins, the ideal therapy may include small doses of statins with phytosterols/phytostanols. The combined treatment will be most efficient in individuals with high cholesterol absorption efficiency.
Individual cholesterol absorption capacity and endogenous production varies due to genetic polymorphism. Response to statins may be determined by the Apo E genotype, by a possible effect on bile acid synthesis and/or by variability in the rate of CYP metabolism.85 There are studies in patients with familial hypercholesterolaemia86 and non-familiar hypercholesterolaemia87 that suggest that statin treatment in those who display an E4 allele reduces LDL-c to a lesser degree than in individuals with E2 or E3 alleles. However, other studies have not been able to confirm this hypothesis.87-90 Individuals with the Apo E4 allele do, nevertheless, absorb more cholesterol than those with other Apo E alleles.91 In a study that measured cholesterol absorption and synthesis in a group of Finns, those with an E2 allele (E2/2, E2/3 or E2/4) absorbed less and synthesised more cholesterol than those with an E3 (E3/3) allele. Individuals with an E4 (E3/4 or E4/4) allele displayed the greatest absorption and lowest synthesis.92 As well as by the Apo E genotype, inter-individual differences in absorption are influenced by polymorphisms of the ABC G5 and G8 genes.91 Differences in the response to sterol esters in mild hypercholesterolaemics have been found to be dependently of ApoE polymorphisms.83 Patients carrying the ApoE4 allele decreased less the total cholesterol, LDL-cholesterol and ApoB levels than their ApoE2 and ApoE 3 carrier counterparts.83
Statin treatment reduces cholesterol precursors and increases serum phytosterol levels,93 particularly in individuals with a high absorption capacity for cholesterol and sterols in general. In the early 1990's, some studies with Pravastatin and Lovastatin reported increases in cholestanol and serum phytosterols,94-96 while others described reductions.97 Serum concentrations of phytosterols decrease with statin treatment for a variable period of time. Some studies indicate that this reduction depends on their pre-treatment absorption levels, treatment duration and the efficacy in inhibiting cholesterol synthesis.85,97 Several studies deal with the effect of sterol and stanol treatment on LDL-c levels in individuals treated with statins (table V).98 The first study that combined statins and stanols (1.5 g/day of sitostanol ester), reported no significant decrease in total cholesterol or LDL-c levels.99 Results of a later study of individuals with non-insulin-dependent diabetes mellitus treated with Pravastatin displayed an additional 6% decrease in LDL-c levels when these patients were given sitostanol esters (3 g/day) in margarine, even when initial cholesterol absorption was already low.100 Even greater reductions (16-20%) were observed in other studies although these were unblinded and uncontrolled.101-102 Reductions in total cholesterol levels of 11% and of LDL-c levels of 16% were obtained in postmenopausal women who had previously suffered myocardial infarction and were taking Simvastatin after have been given a complementary treatment consisting of sitostanol ester (3 g/day) in margarine.101 Serum LDLc levels decreased by 20% in individuals with familial hypercholesterolaemia who were given Simvastatin together with 2.24 g/day of stanols.102 LDL-c reductions of 7-11% were reported in four-to-eight-week-long placebo-controlled, double-blind random studies with phytosterol and/or phytostanol esters.103-107 In one of these studies, 5.1 g/day of phytostanol ester in margarine in conjunction with a stable statin treatment further reduced total serum cholesterol levels by 7% and those of LDL-c by 10%.91 Various studies have shown that phytostanols103 and phytosterols105 have an additive, not a synergic, effect when given together with statins to individuals with primary hypercholesterolaemia.105 The four treatment options of this study were: placebo plus regular margarine, placebo plus phytosterol-enriched (2 g) margarine, cerivastatin (400 μg) plus regular margarine and cerivastatin (400 μg) plus phytosterol-enriched (2 g) margarine. Cerivastatin vs. placebo reduced LDL-c by 32% and enriched margarine vs. regular margarine reduced these levels by 8%. Phytosterol-enriched margarine taken together with cerivastatin induced a 39% reduction but no significant interactive effect between phytosterol and statin was observed. With regard to the reduction of cholesterol levels through phytosterol or phytostanol treatment some of the studies reviewed report no statistical differences between hypercholesterolaemic individuals treated with statins and non-treated hypercholesterolaemics104 or normocholesterolaemic individuals.105.
Few studies compare the efficacy of plant sterols and stanols. The majority, lasting for only 3-4 weeks, reflect insignificant differences in effectiveness. Differences were detected, however, between the administration of phytosterol esters (1.6 g/day) and phytostanol esters (1.6 g/day or 2.6 g/day) over a two-month-long period to healthy individuals and to others with familial hypercholesterolaemia receiving statin treatment.106 The effect of the phytosterols decreased at the end of the study and LDL-c level was not significantly different from baseline. In addition, these individuals displayed an increase in serum phytosterol levels and decrease bile acid synthesis. In contrast, after the two-month-long treatment, phytostanol esters significantly decreased both LDL-c and phytosterol levels, without producing any effect on bile acid synthesis.106
Taking into account all the information previously reviewed, it is possible to conclude that phytostanol esters with respect to phytosterol esters should be preferred for long-term treatment of hypercholesterolaemia. Various studies have confirmed that the additive effect produced by combining phytostanols with a small dose of statins reduces LDL-c levels by approximately 10%,84 this reduction is slightly greater than that achieved by doubling the dose of statins.107 Furthermore, by lowering the dose of statins the risk of rhabdomyolysis, which appears to be dose-dependent, also decreases.108 In addition, a triple therapy including stanol esters, statins and resins can reduce LDL-c by up to 67% and may be effective in the most resistant hypercholesterolaemias. 93
Conclusions and future remarks
This article reviews some outstanding aspects of the interactions between statins and dietary components. The most relevant results were those obtained with grapefruit juice, oily fish and phytosterols. Given the risk of statininduced adverse reactions (e.g. myopathy and rhabdomyolysis), a close monitorisation of patients receiving statin treatment is necessary. Concomitant administration of this group of drugs with certain foods can lead to a reduction (in the case of fibre) or an increase (with grapefruit juice, n-3 fatty acids, phytosterols) of their pharmacological action. Therefore, to optimise the pharmacological treatment, it is necessary to understand and adjust certain important aspects of the diet of these patients. Besides, follow-up studies of the therapeutic effects of statins in patients consuming a Mediterranean diet, which includes high levels of fibre, citric fruits, oily fish, phytosterols, olive oil rich in polyphenols and other minor components, are necessary to assure the correct prescription and dosage of statins in these individuals. To conclude, we emphasize that there are possible interactions that require further investigation, for example, different types of oils -e.g. poor and rich in minor compounds olive oils-, fruit juices or fibre, or the prolonged consumption of high doses of alcohol.
Acknowledgements
The authors are grateful to the Nutrition and Cardiovascular Disease PhD students for their interest in the problems of the nutrition and drug interactions.
References
1. Florez J, Freijanes J. Fármacos hipolipoproteinemiantes. In: Florez J, Armijo JA, Mediavilla A, eds. Control de la obesidad. Farmacología humana, Masson SA, pp. 950-953, Barcelona, 1997. [ Links ]
2. Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 2006; 112: 71-105. [ Links ]
3. Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet 1997; 32: 403-25. [ Links ]
4. Guía europea para la investigación de interacciones medicamentosas. CPMP/EWP/560/95.1997 [ Links ]
5. Lairon D. Dietary fibres: effects on lipid metabolism and mechanisms of action. Review. Eur J Clin Nutr 1996; 50: 125-33. [ Links ]
6. Richter WO, Jacobs BG, Schwandt P. Interaction between fibre and lovastatin. Lancet 1991; 338: 706. [ Links ]
7. Agrawal AR, Tandon M, Sharma PL. Effect of combining viscous fibre with lovastatin on serum lipids in normal human subjects. Int J Clin Pract 2007; 61: 1812-8. [ Links ]
8. Moreyra AE, Alan CW, Koraym A. Effect of combining psyllium fiber with simvastatin in lowering cholesterol. Arch Intern Med 2005; 165: 1161-6. [ Links ]
9. Expert Panel of Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486-97. [ Links ]
10. Moghadasian MH. Clinical pharmacology of 3-hidroxy-3-methylglutaryl coenzyme A reductase inhibitors. Life Sci 1999; 65: 1329-37. [ Links ]
11. Sánchez-Muniz FJ, Varela P, Bastida S, González JM. Enfermedad cardiovascular, hipertensión arterial, dislipemias. In: Carbajal A, Varela P, eds. Cuidados farmacológicos y nutricionales en el paciente de edad avanzada. Fundación General de la Universidad Complutense, pp. 1-47, Madrid,: 2001. [ Links ]
12. Smit JW, Wijnne HJ, Schobben F, Sitsen A, de Bruin TW, Erkelens W. Effects of alcohol comsumption on pharmacockinetics, efficacy and safety of fluvastatin. Am J Cardiol 1995; 76: 89A-96A. [ Links ]
13. Smit JW, Wijnne HJ, Schobben F, Sitsen A, de Bruin TW, Erkelens W. Effects of alcohol and fluvastatin on lipid metabolism and hepatic function. Ann Int Med 1995; 122: 678-80. [ Links ]
14. Bailey DG, Spencer JD, Edgar B, Bayliff CD, Arnold JM. Ethanol enhances the hemodynamic effects of felofipine. Clin Invest Med 1989; 2: 357-62. [ Links ]
15. Garvan CK, Lipsky J. Drug-grapefruit juice interactions. Mayo Clin Proc 2000; 75: 933-42. [ Links ]
16. Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther 1998; 64: 477-8. [ Links ]
17. Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther 1999; 66: 118-27. [ Links ]
18. Lilja JJ, Neuvonen M, Neuvonen PJ. Effects of regular consumption of grapefruit juice on the pharmacokinetics of simvastatin. Br J Clin Pharmacol 2004; 58: 56-60. [ Links ]
19. Fukazawa I, Uchida N, Uchida E, Yasuhara H. Effects of grapefruit on pharmacokinetics of atorvastatin and pravastatin in Japanese. Br J Clin Pharmacol 2003; 57: 448-55. [ Links ]
20. Ando H , Tsuruoka S, Yanagihara H, Sugimoto K, Miyata M, Yamazoe Y et al. Effects of grapefruit juice on the pharmacokinetics of pitavastatin and atorvastatin. Br J Pharmacol 2005; 60: 494-7. [ Links ]
21. Reamy BV, Stephens MB. The grapefruit-drug interaction debate: role of statins. Am Fam Physician 2007; 76: 190-2. [ Links ]
22. Stumpt AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician 2006; 74: 605-8. [ Links ]
23. Rogers JD, Zhao J, Liu L. Grapefruit juice increases felodipine oral availability by decreasing intestinal CYP3A protein expression. J Clin Invest 1997; 99: 2545-53. [ Links ]
24. Lilja JJ, Kivistö KT, Neuvonen PJ. Duration of effect of grapefruit juice on the pharmacokinetics of the CYP3A4 substrate simvastatin. Clin Pharmacol Ther 2000; 68: 384-90. [ Links ]
25. Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther 2007; 81: 495-502. [ Links ]
26. Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H et al. Citrus juices inhibit the function. Drug Metab Dispos 2005; 33: 518-23. [ Links ]
27. Li P, Callery PS, Gan L-S, Balani SK. Estearase inhibition attribute of grapefruit juice leading to a new drug interaction. Drug Metab Dispos 2007; 35: 1023-31. [ Links ]
28. Li P, Callery PS, Gan L-S, Balani SK. Estearase inhibition by grapefruit juice flavonoids leading to a new drug interaction. Drug Metab Dispos 2007; 35: 1203-8. [ Links ]
29. De Castro WV, Mertens-Talcott S, Derendorf H, Butterweck V. Grapefruit juice-drug interactions: Grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J Pharm Sci 2007; 96: 2808-17. [ Links ]
30. Uesawa Y, Mohri K. The use of heat treatment to eliminate drug interactions due grapefruit juice. Biol Pharm Bull 2006; 29: 2274-8. [ Links ]
31. Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 2005; 33: 518-23. [ Links ]
32. Koitabashi Y, Kumai T, Matsumoto N, Watanabe M. Orange juice increased the bioavailability of pravastatin, 3-hydroxi-3-methylglutaryl CoA reductase inhibitor, in rats and healthy human subjects. Life Sci 2006; 78: 2852-9. [ Links ]
33. Sorokin AV, Duncan B, Panetta R, Thompson PD. Rhabdomyolysis associated with pomegranate juice consumption. Am J Cardiol 2006; 98: 705-6. [ Links ]
34. Keys A. Coronary heart disease global picture. Atherosclerosis 1975; 22: 149-92. [ Links ]
35. Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res 1985; 26: 194-202. [ Links ]
36. Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R et al. The diet and 15 years death rate in the seven countries. Am J Epidemiol 1986; 124: 903-15. [ Links ]
37. De Roos NM, Boots ML, Siebelink E, Schouten E, Katan MB. Flow-mediated vasodilation is not impaired when HDL-cholesterol is lowered by substituting carbohydrates for monounsaturated fat. Br J Nutr 2001; 86: 181-8. [ Links ]
38. Montoya MT, Porres A, Serrano S, Fruchart JC, Mata P, Gerique JAG, et al. Fatty acid saturation of the diet and plasma lipid concentrations, lipoprotein particle concentrations, and cholesterol efflux capacity. Am J Clin Nutr 2002; 75: 484-91. [ Links ]
39. Dietschy JM. Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations. J Nutr 1998; 128: 444S-448S. [ Links ]
40. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992; 12: 911-9. [ Links ]
41. Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr 1997; 65 (5 Suppl.):1628S-1644S. [ Links ]
42. Sánchez-Muniz FJ, Bastida S, Gutiérrez-García O, Carbajal A. Olive oil-diet improves the simvastatin effects respect to sunflower oil-diet in men with increased cardiovascular risk. A preliminary study. Nutr Hosp 2009; 24: 333-9. [ Links ]
43. Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA 2007; 298: 776-85. [ Links ]
44. Sung M, Kim I, Park M, Whang Y, Lee M. Differential effects of dietary fatty acids on the regulation of CYP2E1 and protein kinase C in human hepatoma HepG2 cells. J Med Food 2004; 7: 197-203. [ Links ]
45. Stupans I, Stretch G, Hayball P. Olive oil phenols inhibit human hepatic microsomal activity. J Nutr 2000; 130: 2367-70. [ Links ]
46. Stupans I, Murray M, Kirlich A, Tuck KL, Hayball PJ. Inactivation of cytochrome P450 by the food-derived complex phenol oleuropein. Food Chem Toxicol 2001; 39: 1119-24. [ Links ]
47. Maggie B, Covington MD. Omega-3 fatty acids. Am Fam Physician 2004; 70: 133-40. [ Links ]
48. Gruppo Italiano per lo Studio della Sopravvivenza nell`Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999; 354: 447-55. [ Links ]
49. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 2002; 112: 298-304. [ Links ]
50. Panagiotakos DB, Pitsavos C, Zampelas A, Chrysohoou C, Griffin BA, Stefanadis C et al. Fish consumption and the risk of developing acute coronary syndromes: the CARDIO2000 study. Int J Cardiol 2005; 102: 403-9. [ Links ]
51. Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort. I. Circulation 2006; 113: 195-202. [ Links ]
52. Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis. Lancet 1978; 2: 117-9. [ Links ]
53. Terrano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamaru Y et al. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis 1983; 46: 321-31. [ Links ]
54. Fox PL, DiCorletto PE. Fish oils inhibit endothelial cell proliferation of platelet-derived growth factor like protein. Science 1988; 241: 453-6. [ Links ]
55. Hostmark AT, Bjerkedal T, Kierulf P, Flaten H, Ulshagen K. Fish oil and plasma fibrinogen. BMJ 1988; 297: 180-1. [ Links ]
56. Geleinjse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 2002; 20: 1493-9. [ Links ]
57. Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. Am Coll Cardiol 2005; 46: 120-4. [ Links ]
58. Dester M, Den Ruijter, Geza Berecki, Opthof T, Verkerk AO. Pro and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res 2007; 73: 316-25. [ Links ]
59. Young-Xu Y, Jabbour S, Goldberg R, Blatt CM, Grayboys T, Bilchik B et al. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol 2003; 92: 1379-83. [ Links ]
60. Siu CW, Lau CP, Tse HF. Prevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversion. Am J Cardiol 2003; 92: 1343-5. [ Links ]
61. Mitchell LB, Powell JL, Gillis AM, Kehl V, Hallstrom AP, for the AVID Investigators. Are lipid-lowering drugs also antiarrhythmic drugs? An anlysis of the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J Am Coll Cardiol 2003; 42: 81-7. [ Links ]
62. Kumagai K, Nakashima H, Saku K. The HMG-Co A reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res 2004; 62: 105-11. [ Links ]
63. Liu T, Li G, Korantzopoulos P, Goudevenos JA. Statins and prevention of atrial fibrillation in patients with heart failure. Int J Cardiol 2009;135:e83-e84. Comment on Abi Nasr I, Mansencal N, Dubourg O. Management of atrial fibrillation in heart failure in the elderly. Int J Cardiol 2008; 125: 178-82. [ Links ]
64. Duzendorfer S, Rothbucher D, Schatzberger P, Reinisch N, Kähler CM, Wiedermann CJ. Mevalonate-dependent inhibition of transendotelial migration and chemotaxis of human peripheral blood neutrophils by pravastatin. Circ Res 1997; 81: 963-9. [ Links ]
65. Alberts AW. HMGCo-A reductase inhibitors-the development. Atherosclerosis Rev 1998; 18: 123-31. [ Links ]
66. Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232: 34-47. [ Links ]
67. Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk 1997; 4: 385-91. [ Links ]
68. Martin de Santa-Olalla, Sánchez-Muniz FJ, Vaquero MP. N-3 fatty acids in glucose metabolism and insulin sensitivity. Nutr Hosp 2009; 24: 203-17. [ Links ]
69. Higueras López FJ. Características dietéticas en pacientes dislipémicos con consumo de estatinas en Toledo. Diploma de Estudios Avanzados. Doctorado en Nutrición. Sánchez-Muniz FJ, advisor. Facultad de Farmacia. Madrid: Universidad Complutense. 2007. [ Links ]
70. Higueras FJ, Sánchez-Muniz FJ. Interacción de pescado vs. carne con estatinas en hipercolesterolémicos. X Congreso de la Sociedad Española de Nutrición (SEN) y 1er Simposio de la Fundación Española de la Nutrición (FEN). 2007, Nov 21-24; Segovia, España. [ Links ]
71. Contacos C, Barter PJ, Sullivan DR. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia. Arterioscler Thromb 1993; 13: 1755-62. [ Links ]
72. Nordøy A, Bønaa KH, Nilsen H, Berge RK, Hansen JB, Ingebretsen OC. Effects of Simvastatin and omega-3 fatty acids on plasma lipoproteins and lipid peroxidation in patients with combined hyperlipidaemia. J Intern Med 1998; 243: 163-70. [ Links ]
73. Nordøy A, Bønaa K.H, Sandset P.M, Hansen J, Nilsen H. Effect of w-3 fatty acids and simvastatin on hemostatic risk factors and postprandial hyperlipemia in patients with combined hyperlipemia. Arterioscler Thromb Vasc Biol 2000; 20: 259-65. [ Links ]
74. Nordøy A, Svensson B, Hansen JB. Atorvastatin and omega-3 fatty acids protect against activation of the coagulation system in patients with combined hyperlipemia. J Thromb Haemost 2003; 4: 690-7. [ Links ]
75. Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart 2001; 85: 544-8. [ Links ]
76. Nordøy A, Hansen JB, Brox J, Svensson B. Effects of atorvastatin and omega-3 fatty acids on LDL subfractions and postprandial hyperlipemia in patients with combined hyperlipemia. Nutr Metab Cardiovasc Dis 2001; 11: 7-16. [ Links ]
77. McKenney JM, Swearingen D, Di Spirito M, Doyle R, Pantaleon C, Kling D et al. Study of the pharmacokinetic interaction between simvastatin and prescription omega-3-acid ethyl esters. J Clin Pharmacol 2006; 46: 785-91. [ Links ]
78. Sánchez-Muniz FJ, Canales A, Librelotto J, Nus M. El consumo de fitosteroles ¿un arma de doble filo? Grasas y Aceites 2004; 55: 321-7. [ Links ]
79. Vanhanen H, Miettinen TA. Effects of sitostanol esters, dissolved in dietary oil, on serum cholesterol, plant sterols and cholesterol precursors. Circulation 1991; 84: II-601. [ Links ]
80. Miettinen TA, Puska P, Gylling H, Vanhanen HT, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med 1995; 333: 1308-12. [ Links ]
81. Amundsen AL, Ose L, Nenseter MS, Ntanios FY. Plant sterol ester-enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. Am J Clin Nutr 2002; 76: 338-44. [ Links ]
82. Miettinen TA, Gylling H. Plant stanol and sterol esters in prevention of cardiovascular disease. Ann Med 2004; 36: 126-34. [ Links ]
83. Sánchez-Muniz FJ, Maki KC, Schaefer EJ, Ordovas JM. Serum lipid and antioxidant responses in hypercholesterolemic men and women receiving plant sterol esters vary by apolipoprotein E genotype. J Nutr 2009; 139: 13-9. [ Links ]
84. Miettinen TA, Strandberg TE, Gylling H, for the Finnish Investigators of the Scandinavian Simvastatin Survival Study Group. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients. Arterioscler Thromb Vasc Biol 2000; 20: 1340-6. [ Links ]
85. O'Neill FH, Patel DD, Knight BL, Neuwirth CKY, Bourbon M, Soutar AK et al. Determinants of variable response to statin treatment in patients with refractory familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2001; 21: 832-7. [ Links ]
86. Carmena R, Roederer G, Mailloux H, Lussier-Cacan S, Davignon J. The response to lovastatin treatment in patients with heterozygous familial hypercholesterolemia is modulated by apolipoprotein E polymorphism. Metabolism 1993; 42: 895-901. [ Links ]
87. Ordovas JM, Lopez-Miranda J, Pérez-Jimenez F, Rodríguez C, Park JS, Cole T et al. Effect of apolipoprotein E and A-IV phenotypes on the low density lipoprotein response to HMG CoA reductase inhibitor therapy. Atherosclerosis 1995; 113: 157-66. [ Links ]
88. O'Malley JP, Illingworth DR. The influence of apolipoprotein E phenotype on the response to lovastatin therapy in patients with heterozygous familial hypercholesterolemia. Metabolism 1990; 39: 150-4. [ Links ]
89. De Knijff P, Stalenhoef AF, Mol MJ, Gevers Leuven JA, Smit J, Erkelens DW et al. Influence of apo E polymorphism on the response to simvastatin treatment in patients with heterozygous familial hypercholesterolemia. Atherosclerosis 1990; 83: 89-97. [ Links ]
90. Berglund L, Wiklund O, Eggertsen G, Olofsson SO, Eriksson M, Linden T et al. Apolipoprotein E phenotypes in familial hypercholesterolaemia: importance for expression of disease and response to therapy. J Intern Med 1993; 233: 173-8. [ Links ]
91. Patel MD, Thompson PD. Phytosterols and vascular disease. Atherosclerosis 2006; 186: 12-9. [ Links ]
92. Kesaniemi YA, Ehnholm C, Miettinen TA. Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J Clin Invest 1987; 80: 578-81. [ Links ]
93. Gylling H and Miettinen TA. The effect of plant stanol- and sterol-enriched foods on lipid metabolism, serum lipids, and coronary heart disease. Ann Clin Biochem 2005; 42: 254-63. [ Links ]
94. Vanhanen H, Kesäniemi YA, Miettinen TA. Pravastatin lowers serum cholesterol, cholesterol-precursor sterols, fecal steroids, and cholesterol absorption in man. Metabolism 1992; 41: 588-95. [ Links ]
95. Uusitupa MIJ, Miettinen TA, Happonen P, Ebeling T, Turtola H, Voutilainen E et al. Lathosterol and other noncholesterol sterols during treatment of hypercholesterolemia with lovastatin alone and with cholestyramine or guar gum. Arterioscler Thromb 1992; 12: 807-13. [ Links ]
96. Vanhanen H, Miettinen TA. Pravastatin and lovastatin similarly reduce serum cholesterol and its precursor levels in familial hypercholesterolemia. Eur J Clin Pharmacol 1992; 42: 127-30. [ Links ]
97. Miettinen TA, Gylling H. Effects of statins on noncholesterol sterol levels: implications for use of plant stanols and sterols. Am J Cardiol 2005; 96 (Suppl.): 40D-46D. [ Links ]
98. Thompson GR. Additive effect of plant sterol and stanol esters to statin therapy. Am J Cardiol 2005; 96 (Suppl.): 37D-39D. [ Links ]
99. Vanhanen H. Cholesterol malabsorption caused by sitostanol ester feeding and neomycin in paravastatin-treated hypercolesterolaemic patients. Eur J Clin Pharmacol 1994; 47: 169-76. [ Links ]
100. Gylling H, Miettinen TA. Effects of inhibiting cholesterol absorption and synthesis on cholesterol and lipoprotein metabolism in hypercholesterolemic non-insulin-dependent diabetic men. J Lipid Res 1996; 37: 1776-85. [ Links ]
101. Gylling H, Radhakrishnan R, Miettinen TA. Reduction of serum cholesterol in postmenopausal women with previous myocardial infarction and cholesterol malabsorption induced by dietary sitostanol ester margarine: women and dietary sitostanol. Circulation 1997; 96: 4226-31. [ Links ]
102. Vuori AF, Gylling H, Turtola H, Kontula K, Kentonen P, Miettinen TA. Stanol ester margarine alone and with simvastatin lowers serum cholesterol in families with familial hypercholesterolaemia caused by the FH-North Karelia mutation. Arterioscler Thromb Vasc Biol 2000; 20: 500-6. [ Links ]
103. Blair SN, Capuzzi DM, Gottlieb SO, Nguyen T, Morgan JM, Cater NB. Incremental reduction of serum total cholesterol and low-density lipoprotein cholesterol with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000; 86: 46-52. [ Links ]
104. Neil HA, Meijer GW, Roe LS. Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterolenriched fat spread. Atherosclerosis 2001; 156: 329-37. [ Links ]
105. Simons LA. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. Am J Cardiol 2002; 90: 737-40. [ Links ]
106. O’Neill FH, Brynes A, Mandeno R, Rendell N, Taylor G, Seed M et al. Comparison of the effects of dietary plant sterol and stanol esters on lipid metabolism. Nutr Metab Cardiovasc Dis 2004; 14: 133-42. [ Links ]
107. Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol 1998; 81: 582-7. [ Links ]
108. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004; 109 (Suppl. III): 50-7. [ Links ]
![]() Correspondence:
Correspondence:
Francisco J. Sánchez Muniz.
Departamento de Nutrición y Bromatología I (Nutrición).
Facultad de Farmacia. Universidad Complutense de Madrid.
Plaza Ramón y Cajal, s/n.
28040 Madrid.
E-mail: frasan@farm.ucm.es
Recibido: 30-VI-2009.
Aceptado: 9-VII-2009.