Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.25 no.2 Madrid mar./abr. 2010
Lycopene mediated modulation of 7,12 dimethlybenz (A) anthracene induced hepatic clastogenicity in male Balb/c mice
Modulación mediada por licopeno de la clastogenicidad hepática inducida por 7,12 dimetilbenz (A) antraceno en ratones macho BALB/C
A. Koul, N. Arora and L. Tanwar
Department of Biophysics. Panjab University. Basic Medical Sciences Block. Chandigarh. India.
ABSTRACT
The present study was designed to evaluate the modulatory effects of lycopene against 7, 12 Dimethylbenz (a) anthracene induced clastogenicity and oxidative stress in male Balb/c mice. The animals were divided into four groups; group I served as control (vehicle treated). Animals of group III and IV were administered lycopene orally at a dose of 4 mg/kg body weight for 10 weeks. Groups II and IV were administered DMBA, i.p., at a dose level of 40mg/kg body weight, 48hrs before the sacrifice of animals. Exposure to DMBA clearly induced hepatic cell injury as was evident by an increase in micronucleated cell score, lactate dehydrogenase and alkaline phosphatase activities, and Lipid Peroxidation levels. When the lycopene pre-treated animals were challenged with DMBA, a decrease in micronucleated cell score was observed, which was in corroboration with the observed decrease in LDH and ALP activities and LPO levels. DMBA treatment caused an increase in the oxidative stress with consequent alterations in enzymatic antioxidant defense system. Lycopene pre-treatment boosted the antioxidant defense in group IV. Thus, the antioxidant role of lycopene could be plausible in the protective action conferred by lycopene, enabling it to be used an effective natural free radical scavenger.
Key words: Oxidative stress. Carotenoids. Micronucleus. Antioxidants.
RESUMEN
El presente estudio se diseñó para evaluar los efectos moduladores del licopeno frente a la clastogenicidad y el estrés oxidativo inducidos por 7,12 dimetilbenz(A) antraceno en los ratones macho Balb/c. Se dividió a los animales en cuatro grupos; el grupo I sirvió de control (tratado con vehículo). A los animales de los grupos III y IV se les administró licopeno por vía oral a una dosis de 4 mg/kg de peso corporal durante 10 semanas. Los grupos II y IV recibieron DMBA, i.p., a una dosis de 40 mg/kg de peso corporal, 48 horas antes de ser sacrificados. La exposición a DMBA indujo claramente una lesión de los hepatocitos como se hizo patente por el aumento de la puntuación de células micronucleadas, y las actividades de la lactato deshidrogenasa y la fosfatasa alcalina, así como por los niveles de peroxidación lipídica. Cuando a los animales pre-tratados con licopeno se les expuso a DMBA, se observó un descenso de la puntuación de células micronucleadas, lo que corroboraba el descenso observado de las actividades LDH y ALP y de los niveles de LPO. El tratamiento con DMBA produjo un aumento del estrés oxidativo con las consiguientes alteraciones del sistema de defensa antioxidante. El pre-tratamiento con licopeno aumentó notablemente la defensa antioxidante en el grupo IV. Así pues, el papel antioxidante del licopeno podría ser plausible en la acción protectora conferida por el licopeno, permitiendo usarlo como un eliminador natural eficaz de los radicales libres.
Palabras clave: Estrés oxidativo. Carotinoides. Micronúcleo. Antioxidante.
Introduction
Environmental factors are recognized to play a major role in the etiology of various diseases, including cancer.1 Exposure to environmental pollutants such as polycyclic aromatic hydrocarbons (PAHs) is one of the major risk factors responsible for the onset of various diseases associated with oxidative stress.2 PAHs are a large group of organic compounds with two or more fused aromatic rings that are formed during the incomplete combustion of organic materials such as wood, coal and mineral oil and products derived from them. They are also found in motor vehicle exhaust and tobacco smoke and can be produced by pyrolysis of amino acids, fatty acids and carbohydrates during the cooking process.3 PAHs are indirect acting carcinogens that require metabolic activation to form electrophilic moieties capable of binding to cellular nucleophilic target to initiate their carcinogenic action.4 DMBA, a prototype of PAHs is a potent pro-carcinogen, which on metabolism forms epoxides and other toxic reactive oxygen species consequently leading to chromosomal damage and formation of micronuclei.
For the last couple of decades, natural products derived from plants, fruits, spices, herbs etc have been the main focus of research to ameliorate the threat posed by harmful chemicals, toxins etc from endogenous and exogenous sources.5,6,7 Overwhelming evidence from epidemiological studies indicates that diets rich in fruits and vegetables can be associated with a lower risk of numerous diseases.8,9 Natural products like fruits, vegetables, herbs etc contain several promising chemopreventive compounds such as vitamins, minerals, carotenoids and an array of other phytochemicals.
Tomato (lycopersicon esculentum), a member of Solananaceae family, is consumed widely as a vegetable and as processed tomato products (juice, sauce, soup and ketchup). The active compounds isolated from tomatoes that have antioxidative and anticarcinogenic properties include chlorogenic acid, eugenol, quercetin, rutin, kaempferol, naringenin, alpha and beta carotenes, phytotene, neurosporene and lycopene.10 The prominent carotenoid present in tomato is lycopene. Lycopene has received particular attention as a chemopreventive agent because of its highly efficient free radical scavenging activity.11,12,13
The chemopreventive action of lycopene against several diseases can be attributed to its antioxidant activity. Lycopene adminstration to rats subjected to gentamicin-induced oxidative stress exhibited a protective effect on kidney owing to its antioxidant properties. 14 Lycopene supplementation to cigarette smoke exposed ferrets led to inhibition of lung squamous metaplasia and also induced apoptosis.15 In a study conducted on murine keratinocyte cell lines, it was reported that lycopene supplementation to the tumor cell line caused a marked decrease in the growth properties of the cell line.16 Velmurugan and co-workers in their study have demonstrated that tomato protects against the clastogenic effects of MNNG by decreasing the micronucleated cell score, lipid peroxidation andby enhancing the antioxidant status.17
Keeping in view the above mentioned facts, the present study was designed to evaluate the long term modulatory effects of lycopene on DMBA induced hepatic clastogenicity in male balb/c mice.
Materials and methods
Experimental design
Chemicals: 5,5-dithiobis-2-nitrobenzoic acid (DTNB), reduced glutathione (GSH), oxidized glutathione (GSSG), Bovine Serum Albumin (BSA), Thiobarbituric Acid (TBA), reduced nicotinamide adenine dinucleotide (NADH), reduced nicotinamide adenine dinucleotide phosphate (NADPH) were obtained Sigma Chemical Co. (St Louis, MO,USA). Capsules containing Lycopene were obtained from a recognized pharmaceutical company Gelnova, India. The contents of the capsule were reconstituted in olive oil immediately before oral administration to the animals in order to attain the required dose. Rests of the chemicals were obtained from local firms (India) and were of analytical grade.
Animal model and experimental conditions
Male Balb/c mice (6-8 week old) were procured from the Central animal house of Panjab University, Chandigarh. The animals were housed in polypropylene cages bedded with sterilized rice husk. The animals were given free access to clean drinking water (tap water) and standard animal pellet diet (Ashirwad Industries Kharar, Punjab, India), throughout the experiment. The experimental protocols were approved by the Institutional Ethics Committee and conducted according to the Indian National Science Academy Guidelines for the use and care of experimental animals. After acclimatization to the experimental conditions for one week, the animals were randomly divided into four groups of 6-8 animal each and were administered the following treatments.
Treatment of animals: Group I animals served as the control group and was vehicle treated (olive oil). Group II mice were administered with DMBA (i.p.) at a dose of 40 mg/kg body weight, 48 hrs before sacrifice. Group III animals were administered lycopene orally at a dose of 4 mg/kg body wt, daily for 10 weeks.18 Group IV mice were pre-treated with lycopene orally at a dose of 4 mg/kg body wt daily for ten weeks and were administered DMBA (i.p.) at a dose of 40 mg/kg body wt, 48 hours before sacrificing the animals. Lycopene was dissolved in olive oil. Weekly alterations in the body weight, diet and water consumption were observed for the mice in all groups throughout the experiment. After 48 h of the DMBA injection, the mice were sacrificed and hepatic tissue was obtained.
Micronucleus assay
The micronucleus assay was carried out according to the method described by Schmid, 1975.19 For this, the tissue was washed with chilled homogenizing buffer (24mM Na2-EDTA buffer pH 7.5, containing 75 mM of NaCl), and then homogenized at 500 to 800 rpm. The homogenates were then centrifuged at 7000 rpm for 10 min. The supernatant was removed and fresh homogenizing buffer was poured to re-suspend the pellet. A drop of the suspension was put at one end of precleaned, grease free microscopic slide and was spread using cover slip held at an angle of 45º into a smooth layer. The slides were then air dried in dust free environment for at least 12 h before staining. The hepatocytes were then stained with May & Grunwald for 1-2 min followed by staining with Giemsa for 10 min. The slides were rinsed twice in distilled water dried and then rinsed in methanol. The slides were then cleared in xylene and mounted in DPX. Minimum of 300 cells were counted per mice for the presence of micronuclei using light microscope at 45X.
Biochemical assays
After the completion of the respective treatments the mice were sacrificed by cervical dislocation under light ether anesthesia. The hepatic tissue was obtained and perfused with cold normal saline (0.9% NaCl solution), blotted and then weighed carefully. The hepatic tissue was homogenized in 100mM potassium phosphate buffer (pH 7.4) containing 150 mM KCl to obtain 10% homogenate (w/v). The homogenate was subjected to cold centrifuge at 10,000 x g for 30 minutes and the supernatant (PMS) thus obtained was used for various biochemical estimations. Aliquots of 10% homogenate were kept for estimation of reduced glutathione and lipid peroxidation levels.
Lactate dehydrogenase (LDH) - LDH activity was estimated by determining the rate of oxidation of NADH at 340 nm by the method of Bergmeyer and Bernt et al., (1971).20
Alkaline phosphatase (ALP) - ALP activity was assayed using p-nitrophenyl phosphate as a substrate to yield p-nitro phenol phosphate, whose absorbance is determined at 420 nm by the method of Bermeyer, (1963).21
Reduced glutathione (GSH) - GSH was estimated as the total non-protein sulphydryl group by the method described by Moron et al. (1979).22 Homogenates were immediately precipitated with 0.1 ml of 25% trichloroacetic acid and the precipitate was removed after centrifugation at 1500 × g for 10 min. The free-SH groups were assayed in a total 3 ml volume by adding 2 ml of 0.6 mM DTNB prepared in 0.2 M sodium phosphate buffer (pH 8.0), to 0.1 ml of the supernatant and the absorbance was read 412 nm using a Shimadzu UV-160 spectrometer. GSH was used as a standard to calculate micromole of GSH contents/mg protein.
Glutathione-S-transferase (GST) - GST activity was determined spectrophotometrically according to the procedure described by Habig et al., 1974.23 The reaction mixture (3 ml) contained 2.7 ml of 100 mM potassium phosphate buffer (pH 6.5), 0.1 ml of 30 mM CDNB and 0.1 ml of 30 mM GSH. After pre-incubating the reaction mixture at 37ºC for 2 min, the reaction was started by the addition of an appropriate amount of the supernatant. The absorbance was followed for 3 min at 340 nm. The specific activity of GST was expressed as μmol of GSH-CDNB conjugates formed/min/mg protein using an extinction coefficient of 9.6 mM-1cm-1.
Lipid peroxidation (LPO) - The assay for lipid peroxidation was performed according to the method of Wills, 1966.24 0.5 ml of 10% tissue homogenate was diluted to 1 ml using Tris-HCl buffer. The samples were then incubated at 37ºC for 2 h. At the end of the incubation period, 1 ml of cold TCA was added and after thorough mixing the reaction mixture was centrifuged at 800 x g for 10 minutes. To 1 ml of supernatant was added 1ml of TBA and the reaction mixture was boiled at 100ºC for 15 minutes. The pink colored complex was formed whose absorbance was read at 532 nm. The amount of MDA formed (index of lipid peroxidation) was calculated using an extinction coefficient of 1.56 x 105 M-1 cm-1for MDA-TBA chromophore and the results are expressed as nanomole of MDA formed /mg of protein.
Catalase - The catalase activity was estimated by measuring the breakdown of hydrogen peroxide at 240 nm according to the method of Luck, 1971.25 To 1.5 ml of the H2O2 buffer added an appropriate amount of PMS (5-20 μl).The blank lacking H2O2 buffer (containing only phosphate buffer) was also run simultaneously. The decrease in OD is monitored at 240 nm for 3 minutes. The catalase activity is expressed as millimole of H2O2 decomposed/min/mg of protein using anextinction coefficient of 0.0394 M-1cm-1.
Superoxide Dismutase (SOD) - Superoxide dismutase activity was estimated according to the method described by Kono, 1978, wherein reduction of nitroblue tetrazolium mediated by superoxide anions generated by photo oxidation of hydroxylamine hydrochloride to blue formazon was measured at 560 nm.26 The activity of superoxide dismutase was expressed as International Units per mg protein (IU/mg protein), where 1 IU is defined as the amount of enzyme inhibiting the increase in optical density by 50%.
Protein content - Protein content of various samples was estimated by the method of Lowry et al., 1951 using BSA as standard.27
Statistical analysis: The data is expressed as Mean ± SD. Statistical significance was analysed by one-way ANOVA followed by Student's Newman Keul Test.
Results
The mice were observed for changes in body weight, diet and water consumption throughout the experiment. Non significant changes were observed in the diet and water consumption by the mice in all the groups studied (Data not shown).
Micronucleus Assay (fig. 1, a, b, c): A significant increase (p < 0.001) in the micronucleated cell score was observed in DMBA treated group when compared to the control group. The micronucleated cell score also increased significantly (p < 0.001) in the lycopene per se group when compared to the control group. A significant decrease (p < 0.001) in micronucleated cell score was observed in Group IV when compared with Group II i.e. when lycopene pre-treated animals were challenged with DMBA exposure. However, a significant increase (p < 0.001) in the micronucleated cell score of Group IV was observed when compared to Group I (control). When group IV was compared with group III a significant decrease (p < 0.01) was observed.
Liver marker enzymes
Alkaline phosphatase and Lactate Dehydrogenase (table I): The activity of liver marker enzymes ALP and LDH increased significantly (p < 0.01; p < 0.001) in DMBA treated animals (Group II) when compared to control animals. No significant changes in ALP and LDH levels were observed in the lycopene per se group and control animals. A significant decrease in ALP (p < 0.01) and LDH (p < 0.001) activities of Group IV (lycopene + DMBA) was observed when compared to Group II animals.
Antioxidant Defense System
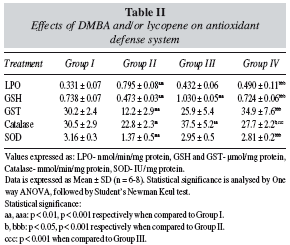
Lipid Peroxidation (table II): A significant increase (p < 0.001) in hepatic LPO level was observed in the DMBA treated animals when compared to the control animals. When lycopene pre-treated animals were challenged with DMBA, a significant decrease (p < 0.001) in hepatic LPO levels was observed when compared to the only DMBA treated group. No significant alteration was observed in the hepatic LPO levels of lycopene per se group and control group.
Reduced Glutathione (table II): DMBA adminstration to animals caused a significant decrease (p < 0.001) in hepatic GSH levels when compared to the control animals. Lycopene per se group exhibited a significant increase (p < 0.001) in hepatic GSH levels when compared to the control group. Group IV animals (lycopene + DMBA) also exhibited a significant increase (p < 0.001) in GSH levels when compared to Group II animals (DMBA).
Glutathione-s-Transferase (table II): GST activity decreased significantly (p < 0.001) in the DMBA treated animals when compared to the control animals. A non significant decrease in GST levels of lycopene per se group was observed when compared to the control animals. However, when lycopene pre-treated animals were challenged with DMBA (Group IV), a significant increase (p < 0.001) in hepatic GST activity was observed when compared to animals treated with DMBA only (Group II).
Catalase and Superoxide Dismutase (table II): DMBA treatment caused a significant decrease in hepatic catalase (p < 0.01) and SOD (p < 0.001) activity when compared to the control animals. Animals of the lycopene per se group exhibited a significant increase (p < 0.01) in catalase activity when compared to the control animals. A significant increase in catalase (p < 0.05) and SOD (p < 0.001) activity in Group IV animals (lycopene + DMBA) was observed when compared to the Group II animals (DMBA). A non-significant change was observed in hepatic SOD activity in the lycopene per se group when compared to the control group.
Discussion
DMBA, a member of PAH class of carcinogens, is present in the environment as a product of incomplete combustion of complex hydrocarbons. Being an indirect carcinogen, DMBA requires metabolic activation to exert its carcinogenic potential. DMBA is metabolized by cytochrome P450 enzymes in the liver to form diol epoxides and other toxic reactive oxygen species. The toxic metabolites of DMBA including diol epoxides, are capable of binding to adenine residues of DNA causing chromosomal damage, thus leading to formation of micronuclei. The micronucleus formed in liver cells is the hall mark of genotoxicity.28.
In the present investigation, micronucleus assay was carried out in order to explore the extent of nuclear damage induced during hepatotoxicity caused by DMBA exposure. DMBA exposure to mice caused a significant increase in the hepatic micronucleated cell score when compared to the control group. These results are in corroboration with previous investigations.29 Mice administered with lycopene also exhibited a marked increase in micro nucleated cell score when compared to the control group. When the lycopene pretreated animals are challenged with DMBA exposure, a significant drop in micronucleated cell score was observed when compared to the group exposed to DMBA only and to the group exposed to lycopene only.
The increase in score of micronucleated cells in lycopene per se group could be attributed to the production of pro-oxidant species of lycopene, resulting from the chronic exposure to lycopene in otherwise normal animals. In the present investigation the observations of potential adverse effects from administration of pure lycopene, with regard to the formation of micronucleus is in line with several human intervention studies, in which adverse effects were induced by pure preparations of carotenoids and phytochemicals at high doses administrated for extended time periods.30,31,32 Breinholt et al, 2003 have reported that prolonged exposure to lycopene induced DNA damage in lymphocytes.33 The deleterious effects of lycopene could be a dose related phenomenon, suggesting that chronic treatment of lycopene should be followed at a low dose level.
In group IV, when animals were challenged with DMBA, after a chronic treatment regime of lycopene, the number of micronucleated cells decreased as compared to group II, which indicates that lycopene exhibited a protective effect when animals are exposed to a carcinogen/genotoxin. Several investigations have revealed that lycopene, was found to be protective against DNA strand breakage induced in wistar rats by the resistant hepatocytes model of hepato-carcinogenesis.34 Bhuvaneswari et al., 2004, in their study on hepatic genotoxicity caused by DMBA, have shown that consumption of tomato extract decreased the micronucleated cell score in DMBA exposed animals relative to the only DMBA treated animals.35 Lycopene is a potent antioxidant because it scavenges free radicals and ROS, consequently offering protection to the cells against carcinogen/genotoxin induced genetic damage.
Lactate dehydrogenase and Alkaline phosphatase are cytotoxic markers that serve as very useful indicators of tissue damage induced by xenobiotics, radiation etc.36 Enhanced activity of ALP and LDH in liver indicates damage to hepatic cells. In the present investigation, damage to hepatocytes by DMBA is reflected by a significant increase in the activities of ALP and LDH in group II when compared to the control animals. Several previous studies support the present observation of carcinogen induced cell damage, with a consequent increase in ALP and LDH activities.37,38 Animals of the lycopene per se group did not exhibit any changes in the ALP and LDH activities when compared to the control group. Pre-treatment of the DMBA challenged animals with lycopene was able to bring down the elevated levels of hepatic ALP and LDH. Some investigations also reported that lycopene was able to reduce the toxicity in liver cells.39
Reactive oxygen species formed during DMBA metabolism can diffuse from the site of generation to other targets within the cells or even propagate the injury outside the cells. Lipid peroxidation causes alterations in membrane integrity, thereby causing impairment of major metabolic functions, which are dependent on membrane structure and integrity.40 Lipid peroxides also causes damage to cellular macromolecules by generating reactive oxygen species that further enhance carcinogenesis.41,42 DMBA exposure significantly elevated the MDA levels in group II animals (about 2 fold) when compared to the control group. Koul et al., 2006 in their study on skin tumor bearing mice have reported that DMBA exposure enhanced the hepatic LPO levels.37 DMBA insult to lycopene pretreated animals showed a marked decline in MDA levels when compared to the only DMBA treated group. Evidences are accumulating in support of the protective role of lycopene.43,44 Among the various defense strategies adopted by lycopene in conferring protection, its antioxidant action seems to be the most plausible one. Likely as other antioxidants, lycopene scavenges reactive oxygen species, singlet molecular oxygen (1O2), and peroxyl radical.12.
Glutathione is a ubiquitous tri-peptide which in its reduced state serves to detoxify electrophiles, maintains the essential thiol states of proteins by preventing the oxidation of SH- groups or by reducing disulphide bonds induced by oxidative stress.45
A noticeable decrease (nearly two fold) in GSH content was observed on DMBA exposure that could be well correlated with the increased levels of lipid peroxidation observed in group II mice. Accumulating literature over the years has made it evidently clear that exposure to carcinogens such as PAH causes a decrease in the GSH levels in the target organs.
A marked increase in hepatic GSH levels was observed in the animals that were on a chronic treatment regime of lycopene. Lycopene pre-treatment to the DMBA exposed animals caused a considerable increase in the hepatic GSH levels, which is in absolute correlation with a drop in MDA levels in the same group. This suggests the ability of lycopene to detoxify cells by up regulating the GSH mediated detoxification process. Administration of tomato alone and in combination with garlic enhanced the GSH levels in liver and stomach of MNNG treated animals, when compared to the animals treated with MNNG only.46
Conjugation reactions in metabolic degradation of xenobiotics are chiefly done by glutathione-S-transferase (GST) in assistance with GSH. GST catalyzes a wide range of reactions in which GSH replaces an easily displaced group in xenobiotic and thus prevents subsequent toxic reactions.47 A significant decrease (more than two folds) in hepatic GST activity was observed in DMBA exposed animals, when compared to the control group. No significant alterations were observed in the hepatic GST activity of lycopene per se group. Lycopene treatment followed by DMBA exposure increased the activity of GST in group IV when compared to group II. Tomato, a rich source of naturally occurring carotenoids has proved to be an effective phase II detoxifier by up regulating the activity of GST under conditions of exposure to carcinogens/genotoxins.33,35
In the present investigation, DMBA induced oxidative stress was associated with decreased activities of catalase and SOD in hepatic tissue. Several studies have repeatedly developed a relation between low levels of antioxidant defense system and exposure to toxins, carcinogens etc. Lycopene treatment to the otherwise normal animals boosted the antioxidant defense system which is evident from an increase in the hepatic catalase activity when compared to the control group, the SOD activity remained unchanged. An increase in the catalase and SOD activities was observed in the lycopene pre-treated and DMBA challenged animals when compared to the animals exposed to DMBA only. Lycopene has been shown to exhibit the highest physical quenching rate constant with ROS.48,13 Reifen et al., 2004 suggested that lycopene as well as 5-aminsalicyclic acid act as antioxidant in oxidative stress against colitis induced iron in rats.49 Srinivasan (2007) reported that lycopene is very efficient ROS scavenging and also has the potential to increase the activity of SOD and catalase.50 Moreira et al., 2005 reported that lycopene rich diet is beneficial in prevention of oxidative damage related to ROS.51
Overall, the present data provides evidence that prolonged exposure to pure lycopene to the normal animals does not affect the liver marker enzymes, LPO and selected antioxidant enzymes in liver. On the other hand this compound was found to increase the number of micronucleated cells in normal animals, which suggested that pure compounds may not prove to be beneficial when given for long duration at this dose. However, pure lycopene had the potential to reduce the number of micronucleated cells when animals were challenged with DMBA, which indicates that it shows some protective role on DMBA exposure. The role of lycopene needs to be investigated further with regard to its dose level.
References
1. Thilly WG. Have environmental mutagens caused oncomutations in people? Nat Genet 2003; 34: 255-9. [ Links ]
2. Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res 2006; 608: 157-62. [ Links ]
3. Collins JF, Brown JP, Alexeeff GV and Samon AG. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol 1998; 28: 45-54. [ Links ]
4. Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug metab and pharmacokin 2006; 21: 257-76. [ Links ]
5. Abdel-Naim AB, Abdel-Wahab MH and Attia FF. Protective effects of Vitamin E and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacol Res 1999; 40: 183-7. [ Links ]
6. Ganger SC, Sandhir R, Rai DV and Koul A. Preventive effects of Azadirachta indica on Benzo(a)pyrene-DNA adduct formation in murine forestomach and hepatic tissues. Phtother Res 2006; 20: 889-95. [ Links ]
7. Steven G, Thomas D, Haffner J, Kroetsch T, Davidson SR, James WE et al. Effect of short-term lycopene supplementation and postprandial dyslipidemia on plasma antioxidants and biomarkers of endothelial health in young, healthy individuals. Vasc Health Risk Manag 2008; 4 (1): 213-22. [ Links ]
8. Steinmetz KA and Potter JD. Vegetables, fruits and Cancer. Cancer Causes Control 1991; 2: 325-57. [ Links ]
9. Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 1992; 18: 1-29. [ Links ]
10. Beecher C. Potential chemopreventive compounds in diet. In chemoprevention of cancer DW Nixon (ed) CRC press, Lond 1995; 21-62. [ Links ]
11. Heber D and Lu QY. Overview of mechanisms of action of lycopene. Exp Biol Med 2002; 227: 920-3. [ Links ]
12. Stahl W and Sies H. Antioxidant activity of carotenoids. Mol Aspects Med 2003; 24: 345-51. [ Links ]
13. Wertz K, Siler U and Goralczyk R. Lycopene: modes of action to promote prostate health. Arch Biochem Biophys 2004; 430: 127-34. [ Links ]
14. Karahan I, Atessahin A, Yilmaz S, Ceribasi AO and Sakin F. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicol 2005; 215: 198-204. [ Links ]
15. Liu C, Lian F, Smith ED, Russell MR and Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up regulating insulin like growth factor binding protein 3 in cigarette smoke exposed ferrets. Cancer Res 2003; 63: 3138-44. [ Links ]
16. Kowalczyk MC, Walaszek Z, Kowalczyk P, Kinjo T, Hanausek M, Slaga TJ. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: implications for skin cancer prevention. Carcinogenesis 2009; 30; 1008-15. [ Links ]
17. Velmurugan B, Bhuvaneshwari V, Abraham SK and Nagini S. Protective effect of tomato against N-Methyl-N'-Nitro-NNitrosoguanidine -induced in vivo clastogenecity and oxidative stress. Nutr 2004; 20: 812-6. [ Links ]
18. Atessahin A, Ceribasi AO, Yilmaz S. Lycopene, a carotenoid, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rats. Basic Clin Pharmacol Toxicol 2007; 100 (6): 372-6. [ Links ]
19. Schmid W. The micronucleus test. Mutat Res 1975; 31; 9-15. [ Links ]
20. Bergmeyer HU and Bernt E (eds). In methods of enzymatic analysis, vol. II. Academic Press: New York 1971; 5574-79. [ Links ]
21. Bergmeyer HU. Methods of enzymatic analysis, Academic press: New York 1963; 783-5. [ Links ]
22. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochem Biophys Acta 1979; 582: 67-9. [ Links ]
23. Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase: The first enzymatic step in mercapturic acid formation. J Biol chem1974; 249: 7130-9. [ Links ]
24. Wills ED. Mechanism of lipid peroxide formation in animal tissue. J Biochem 1966; 99: 667-76. [ Links ]
25. Luck H. In Methods of Enzymatic analysis. Academic Press: New York 1971; 3: 885-94. [ Links ]
26. Kono Y. Generation of Superoxide radical during antioxidation of hydroxylamine and an assay for Superoxide dismutase. Arch Biochem Biophys 1978; 186: 189-95. [ Links ]
27. Lowry OH, Rosebrough NJ, Farr AL and Randall RJ. Protein measurement with Folin Phenol reagent. J Biol Chem 1951; 193: 265-75. [ Links ]
28. Guerin MR. Energy sources of polycyclic aromatic hydrocarbons. Academic Press, New York 1978; 1-42. [ Links ]
29. Suzuki H, Ikeda N, Kobayashi K, Terashima Y, Shimada Y, Suzuki T et al. Evaluation of liver and peripheral blood micronucleus assays with nine chemicals using young rats. A study by the Collaborative Study Group for the Micronucleus test (CSGMT)/Japanese Environment Mutagen Society (JEMS) - Mammalian Mutagenicity Study Group (MMS). Mut Res 2005; 583 (2): 133-45. [ Links ]
30. Lowe GM, Booth LA, Young AJ and Bilton RF. Lycopene and beta carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic Res 1999; 30 (2): 141-51. [ Links ]
31. Patrick L. Betacarotene: the controversy continues. Altern Med Rev 2000; 5: 530-45. [ Links ]
32. Basu A and Imrhan V. Tomatoes verses lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur J Clin Nutr 2007; 61: 295-303. [ Links ]
33. Breinholt VM, Molck AM, Svendsen GW, Daneshvar B, Vingaard AM, Poulsen M et al,. Effects of dietary antioxidants and 2-amino-3-methylimidazo(4,5-f)-quinoline(IQ) on preneoplastic lesions and on oxidative damage, hormonal status, and detoxification capacity in the rat. Carcinogenesis 2003; 41 (10): 1315-23. [ Links ]
34. Toledo LP, Ong TP, Pinho AL, Jordao A, Vanucchi H, Moreno FS. Inhibitory effects of lutein and lycopene on placental glutathione S-transferase-positive preneoplastic lesions and DNA strand breakage induced in Wistar rats by the resistant hepatocyte model of hepatocarcinogenesis. Nutr Cancer 2003; 47 (1): 62-9. [ Links ]
35. Bhuvaneswari V, Velmurugan B, Abraham SK, Nagini S. Tomato and garlic by gavage modulate 7,12-dimethylbenz (a) anthracene-induced genotoxicity and oxidative stress in mice. Braz J Med Biol Res 2004; 37: 1029-34. [ Links ]
36. Reddy AC and Lokesh BR. Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicol 2006; 107:39-45. [ Links ]
37. Koul A, Mukherjee N and Gangar SC. Inhibitory effects of Azadirachta indica on DMBA-induced skin carcinogenesis in Balb/c mice. Mol Cell Biochem 2006; 283: 47-55. [ Links ]
38. Koul A, Binepal G and Gangar SC. Impediment of diethylnitrosamine induced hepatotoxicity in male Balb/c mice by pretreatment with aqueous Azadirachta indica leaf extract. Indian J Exp Biol 2007; 45: 359-66. [ Links ]
39. Floreani A, Baragiotta A, Martines D, Naccarato R, D'odorico A. Plasma antioxidant levels in chronic cholestatic liver diseases. Aliment Pharmacol Ther 2000; 14 (3): 353-8. [ Links ]
40. Sihan S and Turkman G. The effect of Diethylnitrosamine on the levels of Sialic Acid, Lipid- bound Sialic acid and enzyme Activities of Transferase in Rat serum. Turk J Vet Anim Sci 2005; 29: 607-12. [ Links ]
41. Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 1993; 23 (1): 21-48. [ Links ]
42. Bandyopadhyay UJ, Banerjee RK and Das D. Reactive oxygen species oxidative damage and pathogenesis. Current Sci 1999; 77 (5): 658-65. [ Links ]
43. Matos HR, Di Masico P and Medeiros MH. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch Biochem biophys 2000; 383 (1): 56-9. [ Links ]
44. Yilmaz S, Atessahin A, Sahna E, Karahan I and Ozer S. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicol 2006; 218: 164-71. [ Links ]
45. Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 1999; 13: 1169-83. [ Links ]
46. Kumuraguruparan R, Chandra Mohan KVP, Abraham SK and Nagini S. Attenuation of N-methyl-N'-nitro-N-nitrosoguanidine induced genotoxicity and oxidative stress by tomato and garlic combination. Life Sci 2005; 26: 2247-55. [ Links ]
47. Siddiqui MKJ, MahboobMand Mustafa M. Hepatic and extrahepatic glutathione depletion and glutathione-s-transferase inhibition by monocrotophos and thiol analog. Toxicol 1990; 64: 271. [ Links ]
48. Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N and Verma SD. Lycopene attenuates oxidative stress induced experimental cataract development: an in vitro and in vivo study. Nutr 2003; 19(9): 794-9. [ Links ]
49. Reifen R, Nissenkorn A, Matas Z and Bujanover Y. 5-ASA and lycopene decrease the oxidative stress and inflammation induced by iron in rats with colitis. J Gastroentrol 2004; 39 (6): 514-9. [ Links ]
50. Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR and Menon VP. Lycopene as a natural protector against gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochem Biophys Acta 2007; 1770 (4): 659-65. [ Links ]
51. Moreira EA, Fagundes RL, Filho DW, Neves D, Sell F, Bellisle F et al. Effects of diet energy level and tomato powder consumption on antioxidant status in rats. Clin Nutr 2005; 24 (6): 1038-46. [ Links ]
![]() Correspondence:
Correspondence:
Ashwani Koul.
Department of Biophysics.
Basic Medical Sciences Block.
160014 Chandigarh. India.
E-mail: ashwanik@pu.ac.in
Recibido: 27-XI-2009.
Aceptado: 30-XI-2009.