Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.25 no.3 Madrid Mai./Jun. 2010
Nutritional status and eating pattern in prostate cancer patients
Estado nutricional y tipo de alimentación en pacientes con cáncer de próstata
A. Mehdad1, E. McBride1, I. Monteiro Grillo1,2, M. Camilo1 and P. Ravasco1
1Unidade de Nutrição e Metabolismo. Instituto de Medicina Molecular. Faculdade de Medicina da Universidade de Lisboa.
2Serviço de Radioterapia. Hospital Universitario de Santa Maria. Lisboa. Portugal.
ABSTRACT
Background: Prostate cancer is the second most common cancer in men worldwide. Differences in prostate cancer incidence suggest a significant role of environmental factors in the aetiology: obesity, central adiposity and some dietary factors have been suggested as risk factors. This pilot study aimed to analyse the pattern of nutritional status, body fat, and the usual dietary intake among men diagnosed with prostate cancer, consecutively referred to the Radiotherapy Department of the University Hospital Santa Maria.
Patients & methods: Throughout 2006, 87 men with prostate cancer were included. Evaluations: weight & height to calculate body mass index (BMI), waist circumference, percentage body fat with bipolar hand-held bioimpedance analysis (BF-306®), Food Frequency Questionnaire validated for the Portuguese population to assess the usual dietary intake. Frequency analysis and Mann-Whitney U test were used to evaluate prevalence and associations.
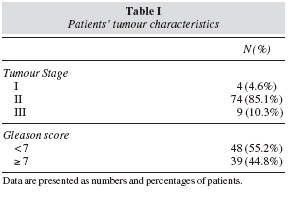
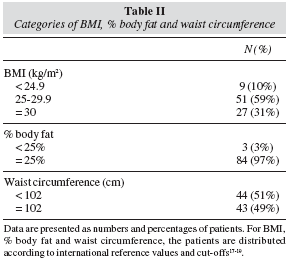
Results: Mean age was 69 ± 7 (46-85) years; 74 (84.1%) patients were in stage II, 5 (5.7%) in stage I & 9 (10.2%) in stage III; 39(45%) patients had a Gleason score ≥ 7. Regarding nutritional status, 78 (89%) patients were overweight/obese, 84 (97%) had a body fat above the maximum limit (> 25%) and 43 (49%) had a waist circumference > 102 cm (prevalence analysis: p < 0.05). Univariate analysis did not show any association between Gleason Score, BMI, %body fat and waist circumference; by multivariate analysis there was an association between higher BMI, %body fat and aggressive Gleason scores (p < 0.002), such variables worsened with age. Food frequency analysis showed a low consumption of n-3 fatty acids sources as well as vegetables and whole grain cereals and a correlation between low yogurt and vegetables intake with more aggressive Gleason scores was found (p < 0.05).
Conclusion: Our findings show a high prevalence of obesity, excessive body and abdominal fat and diets deficient in protective nutrients. Further investigation is warranted as cancer rates in Portugal continue to increase!.
Key words: Prostate cancer. Diet. Obesity. Body fat. Waist circumference.
RESUMEN
Introducción: el cáncer de próstata es el segundo en frecuencia en hombres en el mundo. Las diferencias en la incidencia del cáncer de próstata sugieren un papel significativo de los factores ambientales en su etiología: se ha sugerido la obesidad, adiposidad central y algunos factores dietéticos como factores de riesgo.
Objetivos: este estudio piloto se proponía analizar el patrón del estado nutricional, la grasa corporal y el consumo dietético habitual en hombres diagnosticados de cáncer de próstata y remitidos de forma consecutiva al Servicio de Radioterapia del Hospital Universitario de Santa María.
Pacientes y métodos: a lo largo de 2.006, se incluyeron 87 hombres con cáncer de próstata. Evaluaciones: peso y talla para calcular el índice de masa corporal (IMC), la circunferencia de la cintura, el % de grasa corporal mediante análisis bipolar manual de bioimpedancia (BF-306®), el cuestionario Food Frequency Questionnaire validado en su versión portuguesa para valorar el consumo dietético habitual. Se emplearon los análisis de frecuencia y la prueba U de Mann-Whitney para evaluar la prevalencia y las asociaciones.
Resultados y discusión: la edad media fue de 69 ± 7 (46-85) años; 74 (84,1%) pacientes estaban en estadio II, 5 (5,7%), en estadio I y 9 (10,2%) en estadio III; 39 (45%) pacientes tenían una puntuación de Gleason ≥ 7. Con respecto al estado nutricional, 78 (89%) pacientes eran obesos o tenían sobrepeso, 84 (97%) tenían grasa corporal por encima del límite máximo (>25%) y en 43 (49%) la circunferencia de la cintura era > 102 cm (análisis de prevalencia: p < 0,05). El análisis univariable no mostró ninguna asociación entre la puntuación de Gleason, el IMC, el % de grasa corporal ni la circunferencia de la cintura; el análisis multivariado mostró una asociación entre un mayor IMC, el % de grasa corporal y puntuaciones de Gleason malas (p < 0,002); estas variables empeoraban con al edad. El análisis de frecuencia de alimentos mostró un consumo bajo de fuentes de ácidos grasos n-3 así como de vegetales y de cereales integrales, y se encontró una correlación entre un consumo bajo de yogur y vegetales y unas peores puntuaciones de Gleason (p < 0,05).
Conclusión: nuestros hallazgos muestran un prevalencia elevada de obesidad, exceso de grasa corporal y abdominal y las dietas deficientes en nutrientes protectores.
¡Se requieren investigaciones adicionales puesto que las tasas de cáncer en Portugal siguen aumentando!.
Palabras clave: Cáncer de próstata. Dieta. Obesidad. Grasa corporal. Circunferencia de la cintura.
Introduction
Prostate cancer currently represents the second most common form of cancer in men worldwide, with an increasing incidence rate.1 Both genetic and environmental influences may be involved in its aetiology.
Growing evidence support the integral role of lifestyle factors such as nutrition, obesity, and diet in the prevention or slowed progression of prostate cancer. Obesity is a likely risk factor for several cancers including prostate,2,3 some have reported a positive association2 whereas other did not support those results.4 Central obesity was also associated with an increased risk of prostate cancer5 and with benign hypertrophy.6
Moreover, migrant epidemiological studies have shown that prostate cancer risk increases in men who move from countries with low incidence to Western countries, thereby implying that environmental factors such as nutrition may have a significant impact in cancer development.7 Saturated fat,8 red meat9 and dairy products10 are suggested to be risk factors for prostate cancer; protective factors include micronutrients such as isoflavones,11 lycopene,12 selenium,13 vitamin E14 and vitamin D,15 though results are not consistent.
Based on this framework, this pilot study aimed to assess nutritional status focusing on BMI, % body fat, central obesity as well as in the usual dietary intake of men with prostate cancer, and to explore potential inter-relations between clinical and nutritional factors.
Patients and methods
This pilot cross-sectional study was carried out in the outpatient Radiotherapy Department of the University Hospital of Santa Maria in Lisbon throughout 2006, 87 men with histologically confirmed prostate cancer were consecutively included and evaluated. The study was approved by the University Hospital Ethics Committee and was conducted in accordance with the Helsinki Declaration, adopted by the World Medical Association in 1964, amended in 1975 and updated in 2002. After written informed consent, data were recorded in an individual form pre-constructed for statistical analysis. Data were collected by centrally trained and supervised interviewers. Data included demographics, clinical parameters, anthropometric measures, changes in weight and food intake over the previous 6 months, and the usual dietary intake. The Gleason score, which expresses the severity of prostate tumours according to histology and invasion,16 was obtained from every record; a total Gleason score ≥ 7 is considered histologically aggressive, and Gleason scores < 7 are considered of low aggressiveness.16.
Nutritional parameters
Patients' height was measured using a stadiometer and weight was measured using a SECA® floor scale; measurements were performed in the morning and all patients were in fasting, shoeless and wearing lightweight clothing. BMI was then calculated using the formula [weight(kg)/height(m)2] and classified according to WHO's criteria as: normal weight when 18.5-24.9 kg/m2, overweight if 25-29.9 kg/m2 and obese if ≥ 30 kg/m2.17 Waist circumference was measured midway between the lower margin of the last rib and the iliac crest, in a horizontal plane using a non-stretchable flexible tape. Values were further categorised as normal if < 102 cm and as central obesity if ≥ 102 cm.18 Body percentage of fat mass was determined using a hand-held bipolar bio-impedance instrument (Omron BF306, Omron Healthcare Europe BV, Hoofddorp, The Netherlands); measurements were further categorised into two groups: normal if < 25% and excessive body fat if ≥ 25%.19.
In order to assess usual dietary intake, 77 participants completed a Food Frequency Questionnaire (FFQ) validated for the Portuguese population.20 This comprises a detailed review of the usual intake throughout the previous year in what concerns: types of foods and the frequency of intake per day, week or month, and in what amount; open questions were applied to report and complete the evaluation with all foods not included in the questionnaire but consumed regularly and at least once a week. Further, the foods included in the questionnaire were grouped into broad categories such as dairy products, meat, fish & eggs, fats, sweets & desserts, vegetables, fresh & dried fruits and beverages. Within these categories, respondents' intakes were classified as low (<1-3 times/week), moderate (4-6 times/week) or high (≥ 7 times/week or if consumed daily).
Statistical analysis
Statistical analyses were performed using the SPSS statistical software (SPSS for Windows 14.0). Frequency analysis was used to assess prevalences of nutritional status and also to evaluate food intake. Mann-Whitney U test was used to evaluate associations between parameters of nutritional status, tumour stage and Gleason scores. Bivariate correlations and Spearman correlation coefficient with 2 tailed test of significance, assessed correlations. A concordance analysis using the Kappa coefficient was calculated to measure the rate of agreement between parameters of nutritional status. A multivariate analysis of variance (ANOVA) adjusted for age and for potential errors given by the small sample size, was used to explore associations between various clinical and nutritional variables. Data are presented as numbers and percentages and p values < 0.05 were considered statistically significant.
Results
Patients' mean age was 69 ± 7 (46-85) years and stage II was the most frequent (p < 0.03); additionally, 39 (45%) patients had aggressive tumours with Gleason scores ≥ 7 and 48 (55%) had prostate cancer with low aggressiveness < 7 (table I). Nutritional status revealed that 51 (59%) patients were overweight and 27 (31%) were obese vs only 9 (10%) patients with an adequate BMI (p < 0.001); furthermore, 84 (97%) patients had a % body fat higher than 25% vs only 3 (3%) with a % body fat lower than the higher cut-off limit of 25% (p < 0.001). In 43 (49%) patients, waist circumference was higher than the maximum cut-off value of 102 cm (table II). To confirm consistency of evaluations by all methods, a concordance analysis was performed: we found an agreement between BMI % body fat (Kappa coefficient = 0.93, p < 0.001) and between BMI and waist circumference (Kappa coefficient = 0.44, p < 0.005).
Potential univariate associations between BMI, waist circumference, % body fat, Gleason score and tumour stage was tested through the non-parametric Mann-Whitney U test and Pearson correlation coefficient. No association nor correlation between the Gleason score or tumour stage with nutritional status was found (p < 0.38). However, by multivariate analysis of variance, there was an association between higher BMI, %body fat and aggressive Gleason scores (p < 0.002), and all these variables worsened with age.
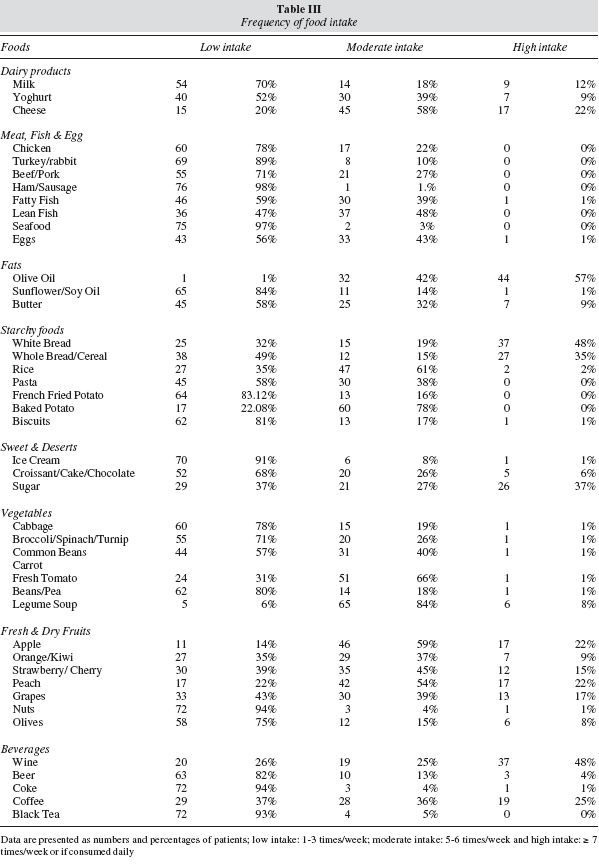
Table III shows patients' poor dietary pattern. The analysis of the reported food frequency of usual intake showed that the majority of patients had low intakes of milk (70%) and yoghurt (52%), cheese was however consumed more often with 58% reporting moderate intake and daily intake in 22%. There was a generalized low intake of vegetables, less than 3 times per week, hence a poor intake of fibre, concomitantly with high and regular intake of simple carbohydrate food sources: 48% of patients consumed white bread on a daily basis; rice and baked potatoes were the next most commonly consumed starch products, with 61% and 78% of patients consuming those between 4-6 times per week respectively. There was also a reduced intake of foods rich in PUFA, mainly from dietary sources of n-3 fatty acids such as nuts (low in 80% of patients) and fatty fish (low in 66% of patients). Overall, the consumption of fresh fruit was moderate (72%). The most popular alcoholic beverage was wine which was consumed on a daily basis by 48% of the patients while25% consumed wine between 4-6 times per week.
Potential associations between different components of the diet and prostate tumour aggressiveness (Gleason score ≥ 7) were explored, but no association was found; however, we did find a correlation between low consumption of yogurt (p = 0.04), broccolis (p = 0.03), cauliflower (p = 0.01) and brussels sprout (p = 0.009) with higher Gleason scores thus more aggressive cancers.
Discussion
In 2004, cancer accounted for more than 13% of all deaths worldwide and the World Health Organisation predicts an increase to almost 18% by 2030.21 In the 1980's, the Europe against Cancer programme fixed the goal of reducing cancer mortality by 15% in Europe by the year 2000. Evaluation of the outcomes of the programme revealed that Portugal and Spain are among a limited number of European countries that showed an increased number of cancer deaths compared to those predicted.22 Nonetheless, the World Cancer Research Fund states that cancer is a preventable disease; a review of the past 30 years of authoritative estimates of the role of food, nutrition and lifestyles in the prevention of cancer, have suggested that approximately 30% of cancers are preventable.1 Prostate cancer is no exception.
Epidemiological studies on the relationship between obesity and prostate cancer are somewhat conflicting: a Swedish cohort study showed a positive association between high BMI and prostate cancer incidence,2 yet the Netherlands Cohort study conducted in 58,000 men over 6 years of follow up, found no overall association.4 In our cross-sectional pilot study of 87 men with prostate cancer, 89% of patients were overweight/obese and 97% of them had a body fat greater than the maximum cut-off value of 25%, and half of these patients had central obesity; however, univariate analysis did not show any association between BMI, central obesity, % fat mass and Gleason score. Although a Type II error cannot be ruled out, when data were examined by a multivariate analysis of variance adjusted for the small sample size, we did find a significant association between higher BMI, increased % body fat and aggressive Gleason scores, and all these variables worsened with age. Additionally, it is noteworthy that the majority of patients did not lose weight over the preceding year. It is tempting to speculate that the link between obesity and prostate cancer may be based on obesity related hormonal changes.23 Excessive central adiposity has been associated with a 3-fold increased risk of prostate cancer and benign hypertrophy; 5,6 moreover serum leptin was higher among men with prostate cancer, and was associated with higher Gleason scores and more advanced tumours.24-26 Obesity is associated with increased levels of insulin-like growth factor (IGF-1), which stimulates cell proliferation27 and higher serum levels of IGF-1 seem to increase the risk of prostate cancer.28 So, obesity per se may be less relevant than associated metabolic changes which may play a role in the progression to more aggressive cancer. Nevertheless, the role of BMI in prostate cancer risk is hard to judge due to the long latency period of the disease; the recorded history of BMI over decades prior to diagnosis might help to determine the real impact of BMI on prostate cancer risk.
Exposure to inadequate diets throughout life may influence prostate cancer progression due to the long pre-clinical stage.1 The literature suggests various associations between dietary intake and risk of prostate cancer: increased intake of calcium and meat with increased risk, whilst high lycopene, vitamin E and selenium intake with a decreased risk.14,29 Some support an association between saturated fat, red meat and dairy products with increased cancer risk, but results are not consistent.10 Other studies show inverse associations between dietary intake of plant foods including cereals, fruits and vegetables and reduced prostate cancer risk,30 others found no association.31 In the current study, whilst not possible to quantitatively assess nutrients, the analysis of the frequency of food intake demonstrated a low consumption of vegetables, nuts, and selenium, lycopene and phytochemicals rich foods, which are consistent with an increased risk of prostate cancer. We also evaluated potential associations between diet and Gleason score; no association was found, though a positive correlation between tumour aggressiveness (Gleason score ≥ 7) and poor yogurt and vegetable intake was observed.
In conclusion, despite this study many limiting factors, e.g small patient cohort, food intake over the past year may not be indicative of their intake in former decades, we do however consider the results relevant, since this is the first study of nutritional variables among Portuguese men with prostate cancer, the most frequent cancer among men in Portugal. Our findings show an alarmingly high prevalence of excess body weight, body fat and the metabolically more active central obesity concomitantly with unbalanced diets poor in protective foods and nutrients. Furthermore, cancer rates in Portugal have increased by 17% in the 20 year period to 200332 and are not responding to European Health Promotion Initiatives in the same manner as other countries in the European Community. The World Cancer Research Fund have declared almost one third of cancers to be preventable through healthy diet and lifestyles.1 Greater focus on prevention is essential for Health Systems to cope with the financial costs of an ageing population, mainly because prostate cancer predominantly affects men over 50. Future research should therefore focus not only on possible diet related risk factors for cancer, but also on how to encourage the adoption of protective diets and life styles by the general population which would concomitantly help to reduce obesity.
Acknowledgements
We are indebted to the helpful medical, nursing and technical staff of the Radiotherapy Department of the University Hospital of Santa Maria.
References
1. World Cancer Research Fund & American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 2007, AICR: Washington DC. [ Links ]
2. Andersson SO, Bergstrom HA et al. Body size and prostate cancer: a 20 - year follow-up study among 135006 Swedish construction workers. J Nathl Cancer Inst 1997; 89: 385-9. [ Links ]
3. Freedland S. Obesity and prostate cancer: a growing problem. Clin Cancer Res 2005; 11: 6763-6. [ Links ]
4. Schuurman AG, Goldbohm RA, Dorant E. Anthropometry in relation to prostate cancer risk in the Nitherlands Cohort Study. Am J Epidemiol 2000; 151: 541-9. [ Links ]
5. Hsing AW, Deng J, Sesterhenn IA, Mostofi FK, Stanczyk FZ. Body size and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev 2000; 9: 1335-41. [ Links ]
6. Lee S, Min HG, Choi SH, Kim YJ, Oh SW, Kim YJ et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity 2006; 14: 172-9. [ Links ]
7. Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol 1999; 161: 152-5. [ Links ]
8. Kondo Y, Homma Y, Aso Y, Kakizoe T. Promotional effect of two-generation exposure to a high-fat diet on prostate carcinogenesis in ACI/Seg rats. Cancer Res 1994; 54: 6129-32. [ Links ]
9. Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K, Wakabayashi K, Sugimura T, Ito N. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res 1997; 57: 195-8. [ Links ]
10. Torniainen S, Hedelin M, Autio V, Rasinperä H, Bälter KA, Klint A, Bellocco R, Wiklund F, Stattin P, Ikonen T, Tammela TL, Schleutker J, Grönberg H, Järvelä I. Lactase persistence, dietary intake of milk, and the risk for prostate cancer in Sweden and Finland. Cancer Epidemiol Biomarkers Prev 2007; 5: 956-61. [ Links ]
11. Kato K, Takahashi S, Cui L, Toda T, Suzuki S, Futakuchi M, Sugiura S, Shirai T. Suppressive effects of dietary genistin and daidzin on rat prostate carcinogenesis. Jpn J Cancer Res 2000; 91: 786-91. [ Links ]
12. Imaida K, Tamano S, Kato K, Ikeda Y, Asamoto M, Takahashi S, Nir Z, Murakoshi M, Nishino H, Shirai T. Lack of chemopreventive effects of lycopene and curcumin on experimental rat prostate carcinogenesis. Carcinogenesis 2001; 22: 467-72. [ Links ]
13. Clark LC, Dalkin B, Krongrad A, Combs GFJr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol 1998; 81: 730-4. [ Links ]
14. Heinonen O, Albanes D, Virtamo J. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. Journal of the National Cancer Institute 1998; 90: 440-6. [ Links ]
15. Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor of prostate cancer? (Hypothesis). Anticancer Res 1990; 10: 1307-11. [ Links ]
16. Fujikawa K, Sasaki M, Arai Y, Yamabe H, Ogawa O, Yishida O. Prognostic criteria in patients with prostate cancer: Gleason score versus volume weighted mean nuclear volume. Clin Cancer Res 1997; 3: 613-8. [ Links ]
17. WHO 1998. Consultation on Obesity. Report of WHO/NUT/NCD. Geneva, World Health Organization. [ Links ]
18. Lean M. Pathophysiology of obesity. Proc Nutr Soc 2000; 59: 331-6. [ Links ]
19. Baumgartner R. Body composition in healthy aging. Ann NY Acad Sci 2000; 904: 437-48. [ Links ]
20. Barros H, Lopes C, Von HP, Fernando PB, Coelho R, Maciel MJ. Risco de enfarte do miocardio: um estudo comunitario. Descricao de estudo e avaliacao da resposta dos participantes comunitarios. Arq Med 1997; 11: 285-94. [ Links ]
21. Boyle P, d'Onofrio A, Maisonneuve P. Measuring progress against cancer in Europe: has the 15% decline targeted for 2000 come about? Annals of Oncology 2003; 14: 1312-25. [ Links ]
22. Clinton S, Giovannucci E. Diet, Nutrition and Prostate Cancer. Annual Review of Nutrition 1998; 18: 413-30. [ Links ]
23. Pasquali R, Casimirri F, Cantobelli S. Effect of obesity and body fat distrubuition on sex hormones and insulin in men. Metabolism 1991; 40: 101-4. [ Links ]
24. Saglam K, Aydur E, Yilmaz M, Goktas S. Leptin influences cellular differentiation and progression in prostate cancer. J Urol 2003; 169: 1308-11. [ Links ]
25. Statin P, Kaaks R, Johansson R et al. Plasma Leptin is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2003; 12: 474-5. [ Links ]
26. Chang S, Hursting SD, Contois JH et al. Leptin and prostate cancer. Prostate 2001; 46: 62-7. [ Links ]
27. Yu H, Rohan T. Role of insulin-like growth factor family in cancer development and progression. J Nathl Cancer Inst 2000; 92: 1472-89. [ Links ]
28. Shaneyfelt T, Husein R, Bubley G, Mantzoros CS. Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol 2000; 18: 847-53. [ Links ]
29. Chan J, Giovannucci E. Vegetables, fruits, associated micronutrients, and risk of prostate cancer. Epidemiologic Reviews 2001; 23: 82-6. [ Links ]
30. Ornish D, Weidner G, Fair WR, Marlin R et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol 2005; 174: 1065-70. [ Links ]
31. Key TJ, Allen N, Appleby P, Overvad K, Tjonneland A, Miller A et al. Fruits and vegetables and prostate cancer: no association among 1,104 cases in a prospective study of 130,544 men in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2004; 109: 119-24. [ Links ]
32. World Health Organisation 2009. Projections of mortality and burden of disease, 2002-2030. [ Links ]
![]() Correspondence:
Correspondence:
Paula Ravasco.
Unidade de Nutrição e Metabolismo.
Instituto de Medicina Molecular.
Faculdade de Medicina de Lisboa.
Avenida Prof. Egas Moniz.
1649-028 Lisboa. Portugal.
E-mail: p.ravasco@fm.ul.pt
Recibido: 3-VI-2009.
Aceptado: 5-VI-2009.