Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.25 no.4 Madrid jul./ago. 2010
Evaluation of chemopreventive response of two cycloxygenase-2 inhibitors, etoricoxib and diclofenac in rat colon cancer using FTIR and NMR spectroscopic techniques
Evaluación de la respuesta quimiopreventiva de dos inhibidores de la ciclooxigenasa 2, etoricoxib y diclofenaco en el cáncer de colon murino empleando las técnicas espectroscópicas FTIR Y NMR
M. Kaur Saini and S. Nath Sanyal
Department of Biophysics. Panjab University. Chandigarh. India.
ABSTRACT
Non steroidal anti inflammatory drugs (NSAIDs) are efficacious in chemoprevention of colorectal cancer. Therefore, the potential ability of Etoricoxib, a selective cycloxygenase-2(COX-2) inhibitor and Diclofenac, a preferential COX-2 inhibitor are considered in the chemoprevention of 1, 2-dimethylhydrazine (DMH) induced colon carcinogenesis in rat model. DMH was injected s.c. for six weeks while Etoricoxib and Diclofenac were fed daily orally alone and also in combination with an weekly subcutaneous injection of 1,2-dimethylhydrazine dihydrochloride (DMH) to the rats. After the treatment period of 6 weeks the animals were sacrificed by an overdose of ether anesthesia and the colonic tissues were removed and studied by the FTIR and NMR Spectroscopic techniques to evaluate the changes occurring in the lipid bilayer of colonic membrane lipids. The alterations in wave number of FTIR spectra as well as the chemical shifts of NMR spectra were recorded which signify the modulation of membrane lipids during colon carcinogenesis and possible cancer prevention by the oral administration of NSAIDs in an experimental model of chemical induced colon carcinogenesis.
Key words: Colon cancer. Chemoprevention. Etoricoxib. Diclofenac. DMH. FTIR. NMR.
RESUMEN
Los fármacos antiinflamatorios no esteroideos (AINE) son eficaces en la prevención del cáncer colorrectal. Por lo tanto, la capacidad potencial de Etoricoxib, un inhibidor selectivo de la ciclooxigenasa-2(COX-2), y de Diclofenaco, un inhibidor preferencial de la COX-2, se considera en la quimioprevención de la carcinogénesis de colon inducida por 1, 2-dimetilhidracina (DMH) en el modelo murino. Se inyectó s.c. DMH durante 6 semanas a la vez que se administraban diariamente por vía oral Etoricoxib y Diclofenaco solos y en combinación con una inyección s.c. semanal de dihidrocloruro de 1,2-dimetilhidracina (DMH) a las ratas. Después del período de tratamiento de 6 semanas, se sacrificó a los animales mediante una sobredosis de anestesia con éter y se extirpó el tejido colónico para estudio con las técnicas espectroscópicas FTIR y NMR para evaluar los cambios que ocurrieron en la bicapa lipídica de las membranas lipídicas colónicas. Se registraron las alteraciones en el número de onda del espectro FTIR así como las desviaciones químicas del espectro NMR, lo que significa la modulación de los lípidos de membrana durante la carcinogénesis colónica y la posible prevención del cáncer mediante la administración de AINE por vía oral en un modelo experimental de carcinogénesis colónica inducida por un agente químico.
Palabras clave: Cáncer de colon. Quimioprevención. Etoricoxib. Diclofenaco. DMH. FTIR. NMR.
Introduction
Recent evidences indicate that cycloxygenase-2(COX-2) is a main pharmacological target for anticancer therapy for which the use of non-steroidal antiinflammatory drugs (NSAIDs) had been suggested.1,2 The use of NSAIDs was particularly linked to the chemoprevention of colorectal cancer3. Anti-inflammatory actions of the NSAIDs rest on their ability to inhibit the activity of COX enzyme, which in turn results in diminished synthesis of primarily the proinflammatory prostaglandins, PGE2.4-7 Their therapeutic effects may partly be due to their ability to induce modification on physical characteristics of the membrane lipid bilayer.8 There are two COX isoforms; while COX-1 is constitutively expressed in almost all tissues, COX-2 is inducible and results due to the expression of an early immediate response gene. Expression of COX-2 is increasingly induced by growth factors, cytokines, pharmacological agents and also during the consecutive stages of colon and other cancer. The role of this enzyme in colorectal carcinogenesis is particularly well established by Oshima and Taketo (2002) showing that COX-2 deficiency partly suppressed the familial adenomatous polyposis as well as the colon cancer.9.
In the present study, experimental colon carcinogenesis is produced by subjecting the rats to 1, 2-dimethylhydrazine (DMH) which is a colon specific carcinogen and metabolically active in the liver and then delivered to the colon via the blood stream or bile as glucuronide conjugate10. After further activation, it methylates DNA mainly at the N' and O6 positions of guanine.11 DNA adduct formation is considered to be the initiating step in the formation of tumerogenesis.12 The colon cancer chemoprevention has been sought by administering Etoricoxib [5-chloro-6-methyl3-(4-methyl sulphonyl)-phenyl]-2-3 bipyridine which belongs to a new generation of NSAIDs that selectively blocks the action of COX-2, while sparing the action of COX-1. This has the therapeutic advantage of decreasing inflammation at tissues sites, while sparing the erosion of gastrointestinal mucosa due to the cytoprotective function and continued production of prostaglandins via the COX-1 isoform.13 Further, the study employs Diclofenac [2-(2, 6-dicloranilino) phenyl acetic acid] which is a highly effective NSAID in reducing inflammation and also there is some evidence to show that Diclofenac is a dual inhibitor of COX-1 and 2.14 In view of the reported damage of the gastric mucosa and bleeding due to the antiplatelet effects caused by the inhibition of COX-115 and also the unexpected cardiovascular side effects due to the COX-2 inhibition alone16, it may be an attractive option to use a dual COX-1 and 2 inhibitor like Diclofenac, which could be an as effective agent in the cancer regression as the traditional NSAIDs (COX-1 inhibitor) or specific COX-2 inhibitor (coxibs), but does not overtly manifest the specific pathophysiology of inhibition of either of the two enzyme isoforms. The present study therefore seeks to clarify the relative effectiveness of the two NSAIDs, one specific COX-2 inhibitor (Etoricoxib) while the other a preferential COX-2 inhibitor (Diclofenac) in colon carcinogenesis in elucidating the membrane alteration by using the spectroscopic techniques like FTIR and NMR. In our earlier studies, we have shown the chemopreventive effectiveness of NSAIDs in colon carcinogenesis with different cyclooxygenase selectivity.17-22.
Materials and methods
Animals
Six to eight week-old male Sprague-Dawley rats of body weight in the range of 80-100 g were obtained from Central Animal House of Panjab University, Chandigarh. The animals were housed in polypropylene cages, embedded with rice husk and maintained under hygienic conditions on standard animal feed and free access to water. The body weight of rats was recorded every week and any change between the different groups in body weight gain could not be found. Animals were maintained as per the principles and guidelines of the Ethics Committee of Animal care of Panjab University for the use of experimental animals for biomedical research.
Experimental design
Rats were randomly assorted and bodily marked for identification. Sixty rats were divided into the six experimental groups having ten animals in each group as follows:
Group 1: Control (vehicle treated); Group 2: DMH treated; Group 3: DMH + Etoricoxib; Group 4: DMH+ Diclofenac; Group 5: Etoricoxib only; Group 6: Diclofenac only. DMH was given in weekly subcutaneous doses of 30 mg/kg body weight for 6 weeks23. 1, 2-dimethyl hydrazine was obtained from Sigma Chemicals Co. (St. Louis, MO, USA) and prepared fresh every week in 1 mM EDTA saline, pH being adjusted to 7.0 using NaOH solution, immediately before subcutaneous injection. Etoricoxib and diclofenac were obtained from Ranbaxy Research Lab (Gurgaon, India) and freshly prepared in the reported anti-inflammatory dose in 0.5% sodium carboxymethyl cellulose.
The route of administration for NSAIDs was by oral intubation daily alone as well as along with DMH as mentioned above. The dose of the NSAIDs was chosen within the therapeutic anti-inflammatory range as based on the reported ED50 value for the rats; 0.6 mg/kg body weight and 8 mg/kg body weight for Etoricoxib and Diclofenac, respectively24,25. At the end of six week duration the animals of each group were over anaesthetized with ether and sacrificed.
Extraction of lipids
The colonic segment starting from the caecum to the rectal ampulla were removed and flushed clear with chilled physiological saline (NaCl solution, 9g/L). For extraction of lipids by the method of Folch et al, the tissue samples collected were hand homogenized with acid washed sand in chloroform: methanol (2:1)26. To the extract obtained, 0.2 vol KCL (0.9%) was added (20% of the total volume). The contents were mixed thoroughly and allowed to stand overnight so as to separate upper aqueous and lower lipid layers. The lower layer was washed with 2ml chloroform: methanol: 0.9% KCL (3:48:47v/v) and evaporated to dryness at temp below 45oC. The dried lipid was redissolved in a known volume of chloroform: methanol (2:1v/v). The lipid extract thus obtained was stored at -20 oC.
FTIR spectroscopy
Lipids were extracted by the above mentioned method, dried at 37 oC and then redissolved in KBr in the ratio of 5:95. The mixture of lipid and KBr was grounded and pelleted at a pressure of 10-15 tons with the help of a hydraulic pressure machine. The pellets obtained were transferred in a spectrophotometer sample holder and the FTIR spectra recorded in the range of 450-4,000 cm-1 in a Perkin Elmer instrument.
NMR spectroscopy
The dried lipid powder was dissolved in CDCl3 and was taken for analysis by NMR.1H NMR spectroscopy was performed on a Bruker Avance ll 400 MHz spectrometer having a magnetic field strength of 9.4 Tesla.
Results and discussion
Spectroscopy is the technique of using the absorption and emission or scattering of electromagnetic radiation for qualitative and quantitative study of the matter. It has attracted the attention of scientists as one of the methods for the identification and characterization of biological samples such as the membranes. The vibrational spectra of a molecule can provide highly resolved vibrational frequency of various functional groups, which can be obtained by either IR or Raman spectroscopy.27 On the other hand the use of 1H NMR spectroscopic techniques for determining the relative amount of biomolecules (in the membranes) had been reported in normal tissues,28 although very little work had been carried out in the cancerous tissues. In view of the fact that many pathological conditions are caused by defects in lipid metabolism, membrane biosynthesis and assembly, lipid protein interaction in the membrane and the alteration of the functional groups assumes critical significance,29 which however had not been reported before. The present report may therefore constitute an important observation of changes in membrane functional groups as studied by FT-IR and NMR spectroscopy in the process of colon carcinogenesis.
FTIR Spectroscopy
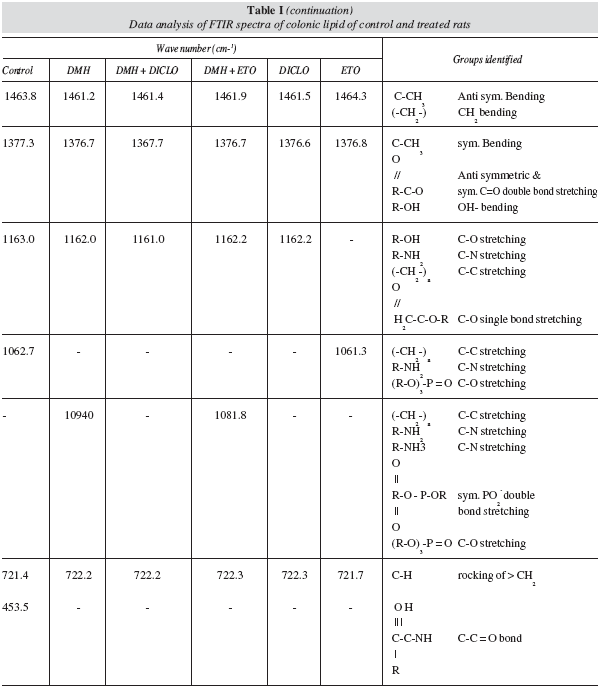
The FT-IR spectra of different treated groups of rat colon are shown in figure 1 (a-f) which table I summarizes the physical characteristics of the spectra which shows considerable changes, also a shift in the wave numbers and peak heights was observed. Groups corresponding to wave number ~3398 are R-OH (OH stretching), R-NH2 (NH2 stretching), R2NH (NH stretching), and also corresponding to wave number ~2922, (-CH2-)n anti symmetric stretching was present in the control group. (-CH2-)n symmetric stretching corresponding to wave number ~2852 was present in all the treated groups. RCO-OR (C = O double bond stretching), C-CH3 anti symmetric bending, and (-CH2-) n CH2 bending were present in all the groups which correspond to the ~1741 and ~1463 wave number, respectively. C-CH3 symmetric bending, RCOO anti symmetric and symmetric C = O double bond stretching, and R-OH (OH- bending) were noticed in all the groups which correspond to the wave number ~1377. C-H rocking of > CH2 corresponding to wave number ~721 was also present in all the groups.
C = X stretching corresponds to wave number ~2364 and observed in DMH and Etoricoxib only. R-CO-OH C = O double bond stretching was present in all the groups except DMH, DMH+ Diclofenac and Diclofenac only which corresponds to the wave number ~1710. RHC = CHR' C = C stretching, RNH2 bending, R2NH (NH bending amide I bond ) was present in Control and Etoricoxib and absent in all other groups corresponding to wave no ~1653. RCOO anti symmetric and symmetric C = O double bond stretching and R2NH (NH bending amide-III bond) were present in DMH+ Diclofenac treated group only corresponding to wave no ~1560. RCOO anti symmetric and symmetric C = O double bond stretching, N (CH3)3 + symmetric CH3 bending and ROH (OH bending) were present in Control and Etoricoxib groups only which corresponds to wave no ~1404. ROH (C-O stretching), RNH2 (C-N stretching), (-CH2-)n (C-C stretching) and -H2C-COOR (C-O single bond stretching) correspond to wave no ~1163 which were present in all the treated groups except Etoricoxib. (-CH2-)n (C-C stretching), R-NH2 (C-N stretching) and (R-O)3-P = O (C-O stretching) were present in Control and Etoricoxib group only which correspond to wave no ~1062.(-CH2-)n (C-C stretching), R-NH2 (C-N stretching, R-NH3 + (C-N stretching), R2PO4 symmetric PO2- double bond stretching and (R-O)3-P = O (C-O stretching) were present in DMH and DMH + Etoricoxib groups and absent in all other groups, corresponding to wave number ~1094 (-CH2-)n.
NMR Spectroscopy
Analytical NMR spectroscopy yields a spectrum of peaks at different chemical shifts. These peaks arise from individual chemical functional groups and thus can be used to determine the identity of an isolated unknown compound. Casu et al. (1992) reported the NMR analysis of lipids extracted from the erythrocytes and plasma of humans.30 Furthermore, 1H NMR spectroscopy has also been applied successfully in the diagnosis of Smith-Lemli-Opitz syndrome (SLOS).31,32 In the present study we used 1H NMR spectroscopy for detecting the changes occurring at lipid composition level during the neoplastic transformations in colon and the NSAIDs induced regression of such tissues. The 400 MHz proton NMR spectra revealed the presence of various metabolites at different chemical shifts values (fig. 2 a-f) (table II). The metabolite peaks observed in our experiment were compared with the peaks obtained in the work done by Oostendorp et a, 2006 on lipid extract from blood plasma of humans.33 The chemical shifts for the groups corresponding to 0.68, 0.86, 0.88, 0.91 and 1.01ppm were found to be similar as reported by these authors with no major alterations. Further, disappearances of peaks were noted corresponding to 1.42-1.55, 1.79-1.88, 1.98-2.09, 2.24-2.35, 2.77-2.87, 4.15/4.29 and 5.26 ppm. 1.42-1.55 ppm proton shift was observed in all the groups except DMH + Diclofenac while 1.79-1.88 ppm proton shift was observed in Diclofenac only and absent in all other groups. Proton shift corresponding to 1.98-2.09 ppm was observed in Control, DMH, DMH + Diclofenac whereas in control, DMH + Diclofenac and Diclofenac only groups, 2.24-2.35 ppm shifts was observed. Unlike the Etoricoxib group which showed the shifts in 2.77-2.87 ppm and Control, the groups of DMH, DMH + Diclofenac and DMH + Etoricoxib observed the shift of 4.15/4.29 ppm. Diclofenac group showed a 5.26 ppm proton shift.
In conclusion the present study was designed to evaluate the anti-inflammatory efficacy of COX-2 preferentially selective NSAID, Diclofenac and COX-2 selective NSAID Etoricoxib, and their possible role in the chemoprevention of colon cancer in DMH induced carcinogenesis where spectroscopic techniques like FTIR and NMR were used to evaluate the changes occurring in the lipid bilayer of colonic membranes under the influence of colon specific carcinogen DMH. FT-IR spectroscopy which is a method of physicochemical analysis, has been employed here to study the macromolecular composition and organization in the biomembranes. The interaction which may exist between membrane lipids and intrinsic proteins and the degree to which intrinsic proteins can perturb a lipid bilayer structure have been the subject of many studies and the present result of changes in the vibration and stretching of functional groups (both symmetric and asymmetric) may support the variations in the structure of membranes at the molecular level due to drug action. Also, the 400 MHz Proton NMR spectra of rat colon revealed the presence of various metabolites and their alterations at different chemical shift values, which may signify the modulation of membrane lipids during the process of carcinogenesis and possible prevention by the NSAIDs. Thus, DMH induced colon carcinogenesis in rat accompanies modification of lipid structures and their functional groups which is corrected by NSAIDs through chemopreventive mechanisms and as such may influence the events leading to the programmed cell death or apoptosis of the cancer cells which may be a COX-2 dependent pathway.
References
1. Thun MJ, Namboodiri MM, Heath CWJ. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991; 325: 1593-6. [ Links ]
2. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci 2003; 24 (2): 96-102. [ Links ]
3. Turner D, Berkel HJ Nonsteroidal anti-inflammatory drugs for the prevention of colon cancer. Can Med Assoc J 1993; 149: 595-602. [ Links ]
4. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nat New Biol 1991; 231: 232-5. [ Links ]
5. Simon IS. Curr Opin Rheumotol 1996; 169-175. [ Links ]
6. Marnett LJ, Kalgutkar AS. Cyclooxygenase inhibitors: Discovery, selectivity and future. Trends Pharmocol Sci 1999; 20: 465-9. [ Links ]
7. Hinz B, Darn CP, Shan TY, Brunek. Antiinflammatory-Antirheumatic drugs. In: Ullman's Encyclopedia of Industrial Chemistry, Sixth edition. Electronic release, Viley VCH, Weinheim. 2000. [ Links ]
8. Lucio M, Ferreira H, Lima JLF, Matos C, Castro BD, Reis S. Influence of some anti-inflammatory drugs in membrane fluidity studied by fluorescence anisotropy measurements. Phys Chem Chem Phys 2004; (6): 1493-8. [ Links ]
9. Oshima M, Taketo MM. COX inhibitor, suppression of polyposis, and chemoprevention. Nippon Yakurigaku Zahhsi 2002; 120 (5): 276-84. [ Links ]
10. Lamont JT, O'Gorman TA. Experimental colon cancer. Gastroenterology 1978; 75: 1157-69. [ Links ]
11. Fiala ES. Investigations into the metabolism and mode of action of the colon carcinogen 1,2-dimethyl hydrazine. Cancer 1975; 36: 2407-12. [ Links ]
12. Hawks A, Magee PN. The alkylation of nucleic acids of rats and mouse in vivo by the carcinogen 1, 2- dimethyl hydrazine. Br J Cancer 1974; 30: 440-7. [ Links ]
13. Gregory LB, Michael HS, Jeffrey GM, Michael JG. Acute renal failure and hyperkalaemia associated with cyclooxygenase-2 inhibitors. Nephrol Dial Transplant 2004; 19: 1149-53. [ Links ]
14. Mazumdar K, Dutta NK, Dastidar SG, Motohashi N, Shirataki Y. Diclofenac in the management of E. coli urinary tract infections. In vivo 2006; 20 (5): 613-9. [ Links ]
15. Vane JR, Bakhle YS, Botting RM. Cyclooxygenase 1 and 2. Ann Rev Pharmacol Toxicol 1998; 38: 97-120. [ Links ]
16. Williams JL, Borgo S, Hasan I et al. Nitric oxide-releasing nonsteroidal anti inflammatory drugs (NSAIDs) alter the kinetic of human colon cancer cell lines more effectively than traditional NSAIDs. Implications for colon cancer chemoprevention. Cancer Res 2001; 61: 3285-9. [ Links ]
17. Mittal N, Kanwar SS, Sanyal SN. Effect of non-steroidal antiinflammatory drugs and the pro-carcinogen 1,2 dimethylhydrazine on the rat intestinal membrane. Nutr Hosp 2008; 23 (5): 439-48. [ Links ]
18. Chopra S, Saini RK, Sanyal SN. Intestinal toxicity of non-steroidal anti-inflammatory drugs with differential cyclooxygenase inhibition selectivity. Nutr Hosp 2007; 22 (5): 528-37. [ Links ]
19. Behal N, Kanwar SS, Sharma P, Sanyal SN. Effect of non-steroidal anti-inflammatory drug etoricoxib on the hematological parameters and enzymes of colon and kidney. Nutr Hosp 2009; 24 (3): 317-23. [ Links ]
20. Saini MK, Kaur J, Sharma P, Sanyal SN. Chemopreventive response of diclofenac, a non-steroidal anti-inflammatory drug in experimental carcinogenesis. Nutr Hosp 2009; 24 (6): 717-23. [ Links ]
21. Nair P, Kanwar SS, Sanyal SN. Effects of non-steroidal antiinflammatory drugs on the antioxidant defense system and membrane functions in rat intestine. Nutr Hosp 2006; 21 (6): 638-49. [ Links ]
22. Sood N, Kaushal N, Sanyal SN. Effect of different non-steroidal anti-inflammatory drugs, aspirin, nimesulide and celecoxib on the disaccharide hydrolases and histoarchitecture of the rat intestinal brush border membrane. Nutr Hosp 2008; 23 (4): 326-31. [ Links ]
23. Kanwar SS, Vaiphei K, Nehru B, Sanyal SN. Antioxidative effects of non-steroidal anti-inflammatory drugs during the initiation stages of experimental colon carcinogenesis in rats. J Environ Pathol Toxicol Oncol 2008; 27 (2): 89-100. [ Links ]
24. Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther 2001; 296: 558-66. [ Links ]
25. Kaur J, Sanyal SN. Association of P13-Kinase and Wnt signaling in non-steroidal anti-inflammatory drugs- induced apoptosis in experimental colon cancer. J Biomed Sci 2009; 1 (4): 395-405. [ Links ]
26. Folch J, Lees M, Sloane-Stanely GH. A simple method for the isolation and purification of total lipids from nervous tissue. J Biol Chem 1957; 226: 497-509. [ Links ]
27. Mathies RA. Biomolecular vibrational spectroscopy. Meth Enzymol 1995; 246: 377-89. [ Links ]
28. Mantsch, HH, McElhaney RN. Phospholipid phase transition in model and biological membranes as studied by infrared spectroscopy. In: Chemistry and Physics of Lipids 1991; 57: 213-26. [ Links ]
29. Ala-Korpela M. 1H NMR spectroscopy of human blood plasma. Prog Nucl Magn Reson 1995; 27: 475-554. [ Links ]
30. Casu M, Lai A, Pilia A, Casti G, Zedda S, Gibbons WA. One and two dimensional 1H-NMR analysis of lipids extracted from erythrocytes and plasma of humans. Arch Gerontol Geriatr 1992; (3) (Supl.): 111-22. [ Links ]
31. Ruan B, Wilson WK, Pang J, Gerst N, Pinkerton FD, Tsai J. Sterols in blood of normal and Smith-Lemli-Opitz subjects. J Lipid Res 2001; 42: 799-812. [ Links ]
32. Xiong Q, Ruan B, Whitby FG, Tuohy RP, Belanger TL, Kelley RI. A colorimetric assay for 7-dehydrocholesterol with potential application to screening for Smith-Lemli-Opitz syndrome. Chem Phys Lipids 2002; 115: 1-15. [ Links ]
33. Oostendorp M, Engelke UFH, Willemsen MAAP, Wevers RA. Diagnosing inborn errors of lipid metabolism with proton nuclear magnetic resonance spectroscopy. Clin Chem 2006; 52 (7): 1395-405. [ Links ]
![]() Correspondence:
Correspondence:
S. Nath Sanyal.
Professor.
Department of Biophysics.
Panjab University.
160 014 Chandigarh. India.
E-mail: sanyalpu@gmail.com
Recibido: 15-XII-2009.
Aceptado: 25-XII-2009.