Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.26 no.5 Madrid sep./oct. 2011
Benefits of blackberry nectar (Rubus spp.) relative to hypercholesterolemia and lipid peroxidation
Beneficios del néctar de mora (Rubus spp.) en relación con la hipercolesterolemia y la peroxidación lipídica
P. R. Ferreira de Araujo1, V. da Silva Santos2, A. Rodrigues Machado2, C. Gevehr Fernandes3, J. A. Silva1 and R. da Silva Rodrigues2
1Department of Agroindustrial Science and Tecnhology. College of Agronomy Elisen Maciel.
2Department of Food Science. College of Home Science,
3Department of Animal Pathology. College of Veterinary Medicine. Federal University of Pelotas. Rio Grande do Sul. Brazil
To CAPES and CNPq (Brazil) for funding and scholarships granted to employees and volunteers who participated in this study.
ABSTRACT
Introduction: In humans, the normal metabolic activity produces free radicals that constantly, along with other risk factors, including hypercholesterolemia may be responsible for the onset of degenerative diseases. Some bioactive compounds present in blackberry (Rubus spp.) have the ability to act as natural antioxidants can make the food to minimize effects on the body caused by reactive oxygen species.
Objective: This study verified the benefits of blackberry nectar through the quantification of triglycerides, total and fraction cholesterol HDL (high density lipoprotein) and LDL-cholesterol (low density lipoprotein), blood glucose and lipid peroxidation in hypercholesterolemic hamsters.
Methods: Two groups were treated with hypercholesterolemic diets (0.1% cholesterol), one of them receiving an additional 5 mL of nectar daily, and a third (control group) treated only with a standard diet. In the blood the quantification of lipids, blood glucose and lipid peroxidation was performed. In the brain, liver and small intestine the lipid peroxidation was determined and in other organs, histopathological evaluations were carried out.
Results: The blackberry nectar reduced the triglycerides serum levels, total cholesterol and LDL-cholesterol in hypercholesterolemic hamsters, without influencing the HDL and blood glucose concentrations. A decrease in the initiation of lipid peroxidation reactions in the blood, brain and small intestine was also observed. Only the liver showed histopathological changes (steatosis), due to excess cholesterol, with no positive influence from the nectar.
Key words: Antioxidant capacity. Lipid serum. Hamster.
RESUMEN
Introducción: En los seres humanos, la actividad metabólica normal produce radicales libres que constantemente, junto con otros factores de riesgo, incluyendo la hipercolesterolemia puede ser responsable de la aparición de enfermedades degenerativas. Algunos de los compuestos bioactivos presentes en mora (Rubus spp.) tienen la capacidad de actuar como antioxidantes naturales pueden hacer que los alimentos para minimizar los efectos sobre el cuerpo causados por las especies reactivas de oxígeno.
Objetivo: Verificar los beneficios del néctar de mora a través de la cuantificación de triglicéridos, colesterol total y fracciones HDL (lipoproteína de alta densidad) y colesterol-LDL (liproteína de baja densidad), glucosa en la sangre y la peroxidación lipídica en hamsters hipercolesterolémicos.
Métodos: Dos grupos fueron tratados con dieta hipercolesterolémica (0,1% de colesterol), que consumían diariamente, otros 5 mL de néctar, y un tercero (grupo control) tratados sólo con dieta estándar. En el sangre se realizo la cuantificación del lípidos, glucosa y la peroxidación lipídica. En el cerebro, el hígado y el intestino delgado se determinó la peroxidación lipídica y en otros órganos, evaluaciones histopatológicas.
Resultados: El néctar de mora reduce los triglicéridos séricos, colesterol total y LDL-colesterol en hamsters hipercolesterolémicos, no influyen en los niveles de colesterol HDL y glucosa en la sangre. También se observo la disminución de las reacciones de iniciación de la peroxidación de lípidos en la sangre, el cerebro y el intestino delgado. El hígado, mostraron cambios patológicos (esteatosis), debido al exceso de colesterol, sin la influencia positiva de néctar.
Palabras clave: Actividad antioxidante. Lípido séricos. Hámster.
Abbreviations
CEA: Food efficiency coefficient.
DPPH: 1,1-diphenyl-2-picrylhydrazyl.
HDL: High density lipoprotein.
HE: Hematoxylin-eosin.
LDL: Low density lipoprotein.
MDA: Malondialdehyde.
TBA: Thiobarbituric acid.
TMP: Tetrametoxipropane.
V/V: volume/volume.
WHO: World Health Organization.
W/V: weight/volume.
W/W: weight/weight.
Introduction
The high-cholesterol diet (hypercholesterolemic) is a major risk factor for the onset of cardiovascular disease,1 which have come to represent a major cause of morbidity and mortality throughout the world. According to the World Health Organization (WHO), it is estimated that by 2015 twenty million people will die from cardiovascular diseases, mainly from heart attacks and strokes.2 The problem arises because of cholesterol, one of the main factors related to atherosclerosis - a cardiovascular disease which has a complex and chronic inflammation of the medium and large caliber arteries and is able to exert a pro-oxidant effect. With hypercholesterolemia there is an increase in the level of cholesterol circulating in the body, which leads to an increased production of oxygen and, therefore, free radicals.3 These play an important role in the pathogenesis of degenerative diseases such as atherosclerosis, given the promoted processes of lipid peroxidation, initiating the oxidation of lipoproteins, mainly the LDL (low density lipoprotein). Through the oxidation of LDL, reactive types that can react with oxygen in the endothelium vascular wall are generated4 and thereby trigger lesions that give rise to atherosclerotic processes. Oxidative stress, the consequence of the imbalance between prooxidants and antioxidants in an organism, is considered to play a very important role in the pathogenesis of several degenerative diseases, such as diabetes, cancer and cardiovascular diseases, including atherosclerosis, because it can help to reduce the body´s antioxidant defenses, establishing risk groups due to the strong production of free radicals.5
It is widely accepted that the consumption of fruits and their derived products may prevent diseases related to the oxidative processes due to the large amount of antioxidants present in these foods, especially vitamin C, carotenoids, selenium and polyphenolic compounds. 6 The blackberry (Rubus spp.) is an excellent source of polyphenolic compounds with a high amount of anthocyanins and good supply of phenolic acids, flavonoids and other non-anthocyanin flavonoids.7 A considerable body of research has focused on the antioxidant capacity of the same and their by-products in systems in vivo. The fruit has anthocyanins above 100 mg/100 g, reaching up to 200 mg/100 g depending on the cultivar (Tupy-112 mg/100 g and Guarani-190 mg/100 g),8 as well as high content of polyphenolic compounds total, which can range up to 500 mg/100 g.9 Ratings chemical with the nectar of blackberry show that the product, as well as fruit, has considerable antioxidant capacity, containing about 170 mg of polyphenolic compounds10 and 75 mg of anthocyanins8 in 100 g of the product corresponding to an antioxidant potential of approximately 80% reduction of DPPH radical (radical 1,1-diphenyl-2-picrylhydrazyl).10
However, the bioactive substances present may not have the same in vivo activity measured using analytical techniques, which often may not be fully available or be rapidly metabolized and excreted, making it ineffective. In the body the active substances are absorbed and metabolized, and may lose their activity or even increase it. Factors such as solubility of the compounds among the different environments of the body, medium pH and concentration of substances can influence the metabolism throughout the gastrointestinal tract. It is therefore important to test the effects of the bioactive substances can have in in vivo systems, because in those environments activity of the compounds absorbed or even its metabolites, which do not always have the same capacity to act as at its origin, was observed.11
With this as the goal, this study was to evaluate the possible beneficial effects of blackberry nectar in living organisms, through the quantification of triglycerides, total and fractional (HDL and LDL) cholesterol, blood glucose and lipid peroxidation in hypercholesterolemic hamsters.
Materials and methods
Blackberry nectar
The product was prepared by mixing, at room temperature, blackberry pulp with mineral water at a ratio of 1:1 (w/w) and additional sucrose (crystallized sugar) to 13o Brix, according to Leitão.10 We used blackberry (Rubus spp.) of the Tupy variety, 2007/2008 crop, grown in the southern state of Rio Grande do Sul (Brazil), pulped in a mechanical extractor with a mesh of 0.8 mm. The nectar was kept in a freezer (-18 ± 2oC) throughout the experiment and thawed the night before administration to the animals. The concentration (mg/100g nectar) of bioactive compounds in the blackberry nectar was determined as follows: phenolic compounds 191.19 ± 0.01, anthocyanins 118.95 ± 0.06, vitamin C 10.78 ± 0.26.12
Animals and experimental design
Twenty-one male hamsters (Mesocricetus auratus) from the Golden Syrian line were used at 25 days, weighing approximately 45 g and were obtained from the Central Animal Facility at the Federal University of Pelotas (Brazil). The animals were kept in individual polypropylene cages at a temperature of 25 ± 2oC, with a light/dark cycle of 12 hours and with free access to food and water. The experimental procedures were approved by the Ethics and Animal Experimentation committee at the Federal University of Pelotas (Brazil) and the animals received human care in accordance to the principles of the 3Rs, introduced in The principles of humane experimental technique.13 After an adjustment period of 5 days they were randomly divided into 3 groups of 7 animals and the feeding ration was initiated, with or without the added cholesterol (0.1%) and with or without blackberry nectar. The experimental groups were the following treatments: Control group: 13 g daily of commercial feed plus 0.3% choline bitartrate (w/w), Cholesterol group: 13 g daily of commercial feed plus 0.3% choline bitartrate and 0.1% crystalline cholesterol (w/w); Nectar group (cholesterol + drink): 13 g daily of commercial feed plus 0.3% choline bitartrate and 0.1% crystalline cholesterol (w/w) + 5 mL of blackberry nectar. The choline bitartrate was added to the diet in order to increase the mobility of the lipids in the body.
Euthanasia
At the end of the experiment (98 days) the animals were anesthetized with inhalation anesthesia with ether in a pan and blood samples were collected by cardiac puncture with the aid of disposable syringes. The blood was centrifuged at 1000 x g for 15 minutes at 4oC so that the plasma was separated from the serum, the latter being stored at -12oC. The night before the euthanasia, the hamsters were deprived of food for a period of 12-14 hours for there to be a fasting condition at the time of sacrifice. After being sacrificed the animals had their organs and tissues (small and large intestine, pancreas, spleen, kidneys, lungs, heart, aorta, brain and liver) removed, with the exception of the small intestine, brain and the liver (wrapped in ultrafreezer foil and frozen at -80oC for lipid peroxidation evaluation), preserved in 10% buffered formalin (v/v) for subsequent histopathological evaluation.
Blood analysis
Blood glucose was measured immediately after blood collection through direct reading equipment in Accutrend GCT (Roche Laboratories of Brazil®), and the results expressed in mmol/L of plasma. Triglycerides, total cholesterol and HDL-cholesterol (high density lipoprotein) were measured in serum by enzymatic techniques using commercial kits called, respectively, Liquiform triglycerides, and HDL Cholesterol Liquiform LE (Labtest Diagnostics® SA, Lagoa Santa- Minas Gerais, Brazil) and were expressed in mmol/L of serum. The LDL-cholesterol was calculated using the Friedewald formula, according to Cordova et al.14, and expressed in mmol/L of serum:
LDL cholesterol = total cholesterol - HDL cholesterol - (triglycerides/5)
Lipidic peroxidation
This consisted of measuring the concentration of substances reactive to thiobarbituric acid (TBA) in the serum and organs of the animals (small intestine, brain and liver) according to the methodology described by Winterbourn, Halliwell and Gutteridge15, with modifications. A spectrophotometer Analytikjena AG model Spekol 1300 in the range of wavelength of 532 nm was used and the results expressed in concentration of malondialdehyde (MDA) nmol MDA/mL of serum or organ homogenates, calculated using a standard TMP curve (1,1,3,3 tetrametoxipropane). The acquisition of homogenates consisted weigh 0.3 g of tissue and add potassium phosphate buffer 20 mM with KCl 140 mM, pH 7.4, at a ratio of 1:10 (w/v). The mixture was homogenized and centrifuged at 1,790 x g for 15 minutes at 4oC.
Histopathological assessment
The organs that were removed from the animals (right lobe of the liver, heart, aorta, large intestine, spleen, kidneys, pancreas and lung) were divided into five fragment series each and embedded in paraffin aiming to obtain histopathological sections of 5 µm thickness, which were stained with hematoxylin-eosin (HE)16 and evaluated by light microscopy. The procedures were carried out by the histotechnic and Histochemistry laboratory, Department of Animal Pathology, College of Veterinary Medicine/UFPel (Federal University of Pelotas)-Brazil, and the samples were evaluated without prior knowledge of the groups of animals to which they belonged to or injuries reported at the time euthanasia.
Statistical analysis
The data were analyzed using the analysis of variance test F and Tukey test with a significance level of 5% for the comparison of means, through STATISTICA version 6.0.17 The results were considered significant only for p ≤ 0.05.
Results
Blood analysis
The high content of cholesterol ingested by hamsters (Cholesterol group) made them reach the hypercholesterolemia condition as according to Nistor et al.18 Moreover, this also made the animals have increased triglycerides, HDL and LDL-cholesterol when compared to those that ate the standard diet (Control group) (table I). The increase in cholesterol intake did not cause an influence on blood glucose. The blackberry nectar improved the lipid ratios of the hamsters that ingested it (Nectar group). The product showed a hypocholesterolemic effect by reducing the concentrations of total cholesterol, LDL-cholesterol and triglycerides. Among animals that were fed cholesterol, reductions were 16% of total cholesterol, 44% of LDL and 31% of triglycerides in animals fed the nectar. Thus, the HDL/LDL ratio increased in the group that drank the blackberry nectar (Nectar group) in relation to cholesterol group. No influence was observed in blood glucose in relation to the ingestion of the nectar (table I).
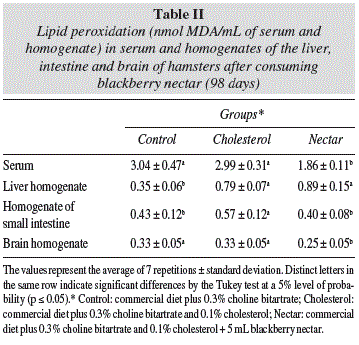
Lipid peroxidation
Analysis of the serum showed that the hypercholesterolemic animals, which consumed the blackberry nectar (Nectar group) were significantly (p ≤ 0.05) less affected by peroxidative processes, a fact which confirms the antioxidant potential of the product in in vivo systems also. The reduction in the levels of MDA (malondialdehyde) in the serum was on the order of 38% in the nectar group (table II). On the analysis of organs (table II), it was not possible to verify the beneficial effect of the blackberry nectar on the liver, but one can observe the effect of cholesterol excess on the body. Compared to the control group (standard diet), the hamsters that ate a diet rich in cholesterol (Cholesterol group) showed a significant (p ≤ 0.05) difference in the lipid peroxidation in the liver, with an MDA (malondialdehyde) index of about 2 times higher, corroborating clinical and experimental evidence that hypercholesterolemia is associated with increased oxidative stress.19 The liver, among the analyzed organs, showed the highest rate of lipid peroxidation in the hypercholesterolemic groups (Cholesterol and Nectar) probably due to the higher accumulation of cholesterol in these animals (table I), which is deposited more intensely in the liver and therefore causes more damage to that organ. In the small intestine the oxidative processes were less intense in hamsters that drank the blackberry nectar, so these animals had their intestine less affected by excess cholesterol. The brain was the organ in which the antioxidant effect was best detected from the blackberry nectar, however it was not possible to verify the interference of cholesterol in the oxidative processes. The Cholesterol and control groups did not differ statistically, but the group of animals that ingested the drink (nectar), showed lower MDA values (malondialdehyde).
Histopathological assessment
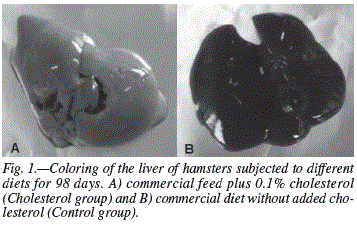
A diet rich in cholesterol triggered changes only in the liver. The hypercholesterolemic animals (Cholesterol and Nectar groups) had injuries caused by steatosis, a result of excessive cholesterol intake through the diet, which causes an excessive intake of fatty acids in the liver (fig. 1). Most samples from the cholesterol group showed discrete and diffused lesions with a predominance of grade 1 injuries in the entire liver. Samples from the Nectar group (hypercholesterolemic animals that drank blackberry nectar) was also higher in the discrete and diffuse lesions, with a higher prevalence of grade 1 injuries. Histopathological studies did not indicate the capacity of the blackberry nectar in protecting the liver from possible diseases caused by high cholesterol, since virtually the same lesions identified in the cholesterol group were found in the Nectar group (animals fed a high-cholesterol diet without the ingestion of nectar). In the heart and aorta there were no changes due to the contribution of cholesterol administered. Samples from the heart did not show endothelial abnormalities or the presence of atheromatous deposits (cholesterol plaques), contrary to what was expected, since the hypercholesterolemic hamsters (Cholesterol and Nectar) showed signs of steatosis caused by excess cholesterol in the diet. Animals in all the experimental groups presented a normal intestine and other organs (kidneys, lungs, pancreas and spleen) showed no significant alterations.
Discussion
The supply of high levels of cholesterol triggered a framework of dyslipidemia in animals that ingested it (cholesterol group) compared to the standard (control), resulting in significantly higher rates (p ≤ 0.05) of total cholesterol, LDL-cholesterol (low density lipoprotein) and triglycerides (table I). The results showed, however, that blackberry nectar has several properties that make it able to reduce the effects of excess cholesterol in the body, as evidenced in the Nectar group (table I, fig. 2).
The average daily consumption of food among the animals did not differ significantly (p ≥ 0.05), getting around 6 g in all experimental groups (table III). Thus, can predict that the decrease in lipemic rates of animals consuming the nectar was not due to a lower hypercholesterolemic feed intake by some animals and yes, the benefits of drinking the blackberry nectar. The food efficiency coefficient (CEA), a result the ratio of total weight gain and total food intake throughout the experiment (98 days) was equivalent for all groups, indicating similarity between them in the conversion of ingested food.
The hypocholesterolemic effect related to blackberry nectar is derived from its rich constitution of antioxidants, characterized by a wide variety of polyphenolic compounds. The benefits of foods rich in such compounds have been reported in different studies. Cherem et al.20 found decreased levels of total cholesterol and LDL cholesterol by about 45 and 54%, respectively, in hypercholesterolemic mice subjected to diets with added eggplant peels, which are rich in anthocyanin compounds. Ribeiro et al.21 evaluated the ability of grape anthocyanin on the plasma constituents of healthy rabbits and found a 17% reduction in total cholesterol levels. The reduction of LDL-cholesterol is extremely important in hypercholesterolemic individuals, because high serum concentrations of lipoproteins are strongly associated with the occurrence of coronary artery diseases such as atherosclerosis, a disease triggered when LDL becomes impermeable to cells and remains in the blood and accumulates in artery walls.22
As for the levels of HDL-cholesterol (high density lipoprotein), there was no difference in relation to the ingestion of blackberry nectar. Ribeiro et al.21 found no influence of grape anthocyanins on plasma levels of HDL-cholesterol of healthy rabbits. Auger et al.23 mentioned the same thing when evaluating the effect of phenolic compounds in red wine on plasma lipids in hypercholesterolemic hamsters. So it is safe to say that the increase in the HDL/LDL occurs because of a decreased rate of LDL-cholesterol which products rich in phenolic compounds are able to provide, and not the increase in HDL. The HDL/LDL ratio is commonly calculated to assess the risk of coronary heart disease. According to Ho et al.24, high LDL-cholesterol showed high atherogenic processes, whereas high levels of HDL-cholesterol have a cardioprotective effect.
Several action mechanisms have been attributed to polyphenolic compounds to explain their effects on lipid metabolism. One of them involves their actions in the increased excretion of bile salts in the stool, and another covers the ability to increase the activity of the microsomal liver system, thereby increasing lipid metabolism.25 Silva et al.26 also reported that flavonoids have the ability to stimulate the activity of lipase, with a reduction in triglyceride levels.
The rich polyphenolic compound formations in blackberry nectar do not influence the rates of blood glucose (table I). Ribeiro et al.21 observed the non-interference of polyphenolic compounds on glucose when administering grape anthocyanin in healthy rabbits.
This study enabled us to verify the antioxidant capacity of the blackberry nectar in in vivo systems, since the product has led to decreased levels of MDA (malondialdehyde), a substance considered as a biomarker of lipid peroxidation reactions in hamsters that ingested it (table II). Rho and Kim27 evaluated the effect of different formulations of grapes (whole grapes, pulp and juice) on lipid peroxidation in the plasma of rats and found that the incorporation of 2% of whole grapes, pulp and juice in the diet promoted a decrease of 10, 17 and 10%, respectively, in lipid peroxidation incidences. Research shows that flavonoids (anthocyanins in particular), a class of polyphenolic compounds present in greater proportions in blackberry nectar, inhibit lipid peroxidation at the initial stage because they act as an antioxidant by removing anions such as superoxide and hydroxyl radicals. It has been proposed that flavonoids interrupt the chain reaction of free radicals, donating hydrogen atoms to peroxyl radicals, with what would be the formation of a flavonoid radical which reacts with the free radical, thus ending the spread of a chain reaction.28
This work also shows that blackberry nectar can act as an antioxidant not only in the blood but also in organs such as the brain, liver and small intestine, protecting them from oxidative processes. The reaction of lipid peroxides with TBA (thiobarbituric acid) has been widely adopted as a sensitive assay method for lipid peroxidation in animal tissues. According to the reaction mechanism, MDA (malondialdehyde - secondary product of lipid peroxidation) is derived from the reaction between TBA and lipid peroxides of polyunsaturated fatty acids with three or more double bonds. Moreover, it is important to emphasize that a reaction pH is the most important factor which affects the reactivity of fatty acid peroxides with TBA.29
A greater ease in determining the rate of lipid peroxidation in the brain possibly occurs due to the fact that, according to Reiter30, it is known to be a more susceptible organ to oxidative damage, mainly due to its high use of oxygen and the high levels of unsaturated lipids and transition metals (like iron) present, besides being an organ with a great deficiency of antioxidant defense mechanisms. However, a lower capacity to inhibit lipid peroxidation in the liver may be due to a lower bioavailability of bioactive substances in the body, than that of a lower activity. For example, if a study showed that less than 1% of the flavonoid quercetin is absorbed in the intestine, where more than 50% of the dose ingested is degraded by colonic microflora, while the remainder is eliminated through feces31, it would leave a percentage too small to act in the protection of various organs of the body. Therefore, the small absorption of some polyphenolic compounds, after ingestion, may justify a lower inhibition of lipid peroxidation in certain organs.
Is also important to emphasize that there are other biomarkers of lipid peroxidation, and MDA, as the enzyme complex naturally present in living organisms. Antioxidant enzymes are capable of scavenging reactive oxygen species and products of lipid peroxidation, thereby protecting cells and tissues from oxidative damage. To prevent oxidative stress, there is an ongoing balance between antioxidants and ROS. When there is an imbalance, ROS may accumulate and trigger oxidative injury by lipid peroxidation, and protein oxidation, accompanied by increased toxic product synthesis and cell death. There is a variety of evidence indicating that antioxidant enzyme activity decreases with increased oxidative stress.32
The lesions detected in the liver of hypercholesterolemic animals (Cholesterol and Nectar groups) prove to be much smaller when compared to other works. Machado33 showed in male hamsters submitted to the diet with a 0.2% cholesterol, incidences of hepatic steatosis grade 4. Factors such as the lowest level of cholesterol added to the diet (0.1%) might have caused minor injuries to the liver. However, it is possible that the addition of choline bitartrate influenced the diet on a larger scale, in the speed of the changes brought about to the liver providing lower levels of steatosis, since this substance, once reinforcing or encouraging the formation of lecithin (factor lipotropics), contributes to the mobilization of hepatic lipids. Several mechanisms have been proposed to explain the role of choline as a lipotropic agent, including its absence, which leads to the impairment of the phospholipid synthesis of lipoproteins, increasing the volume of the liver in regards to fat deposits.34
Conclusions
The results clearly showed that blackberry nectar can provide significant benefits to individuals with hypercholesterolemia. It reduces the levels of triglycerides, total and LDL-cholesterol (low density lipoprotein). Similarly, it has important antioxidant potential in in vivo systems, being able to reduce the initiation reactions of lipid peroxidation in the blood, brain and small intestine. The results shown here are potentially important, as there is a growing interest of world population to consume foods that may help maintain and improve health and reduce the risks of diseases. This work also indicates positive prospects for the ingestion of products ready for consumption, in place of fresh foods, which by the practicality they represent, are becoming increasingly preferred by the current population.
References
1. Praga JM, Thomaz A, Caramelli B. O suco de berinjela (Solarium melongena) não modifica os níveis séricos de lípides. Arq Bras Cardiol 2004; 82: 269-272. [ Links ]
2. World Health Organization. 2009. http://www.who.int/cardiovascular_diseases/en/ (consulted on 14-January-2009). [ Links ]
3. Kok FJ, Van Poppel G, Melse J, Verheul E, Shouten EG, Kruyssen DHCM, Horfman A. Do antioxidants and polyunsaturated fatty acids have a combined association with coronary atherosclerosis? Atherosclerosis 1991; 86: 85-90. [ Links ]
4. Witztum JL. Intensive drug therapy of hypercholesterolemia. Am Heart J 1987; 113: 603-609. [ Links ]
5. Xanthopoulou MN, Fragopoulou E, Kalathara K, Nomikos T, Karantonis HC, Antonopoulou S. Antioxidant and anti-inflammatory activity of red and white wine extracts. Food Chem 2010; 120: 665-672. [ Links ]
6. Perales S, Barbera R, Lagarda MJ, Farré R. Antioxidant capacity of infant fruit beverage; influence of storage and in vitro gastrointestinal digestion. Nutr Hosp 2008;23: 547-553. [ Links ]
7. Antunes LEC. Amora-preta: nova opção de cultivo no Brasil. Cienc Rural 2002; 32:151-158. [ Links ]
8. Mota RV da. Caracterização do suco de amora-preta elaborado em extrator caseiro. Ciênc Tecnol Aliment 2006; 26: 303-308. [ Links ]
9. Antunes LEC, Gonçalves ED, Trevisan R. Alterações de compostos fenólicos e pectina em pós-colheita de frutos de amora-preta. Rev Bras Agrociência 2006; 12: 57-61. [ Links ]
10. Leitão AM. Estabilidade físico-química, microbiológica e sensorial de néctar de amora-preta (Rubus spp.), Cv. Tupy, embalado em polipropileno, no armazenamento. Masters Thesis. College of Agronomy, Pelotas: Federal University of Pelotas, Brazil, 2007. [ Links ]
11. Horst MA, Lajolo FM. Biodisponibilidade de compostos bio-ativos de alimentos. 2009. http://www.fcf.usp.br [consulted on 10-February-2009] [ Links ].
12. Araujo PF de, Rodrigues R da S, Machado AR, Santos V da S, Silva JA. Influência do congelamento sobre as características físico-químicas e o potencial antioxidante de néctar de amora-preta. B CEPPA 2009; 27: 199-206. [ Links ]
13. Russell WMS, Burch RL. The Principles of Humane Experimental Technique. London: Methuen. 1959. p.238. [ Links ]
14. Cordova CMM de, Schneider CR, Juttel ID, Cordova MM de. Avaliação da dosagem direta do col esteral-LDL em amostras de sangue de 10.664 pacientes em comparação com o uso da fórmula de Friedewald. Arq Bras Cardiol 2004; 83:476-481. [ Links ]
15. Winterbourn CC, Gutteridge JM, Halliwell B. Doxorubicin-dependent lipid peroxidation at low partial pressures of O2. J Free Radie Biol Med 1985; 1:43-49. [ Links ]
16. Behner OA, Tolosa EMC, Freitas Neto AG. Manual de técnicas para a histología normal e patológica. São Paulo: EDART. 1976.p.239. [ Links ]
17. STATISTICA for Windows - release 6.0 A. Tula: Statsoft Inc., 2001. [ Links ]
18. Nistor A, Bulla A, Filip DA, Radu A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis 1987; 68: 159-173. [ Links ]
19. Violi F, Loffredo L, Musella L, Marcoccia A. Should antioxidant status be considered in interventional trials with antioxidants? Heart 2004; 90: 598-602. [ Links ]
20. Cherem AR, Tramonte VLCG, Fett R, van Dokkum W. Efeito da casca da berinjela (Solanum melongena) sobre as concentrações plasmáticas de triglicerideos, colesterol total e frações lipidicas em cobaias (Cavia porcellus) hiperlipidêmicos. Rev Bras Pl Med 2007;9:51-60. [ Links ]
21. Ribeiro JN, Oliveira TT de, Nagern TJ, Flores AV. Avaliação da toxicidade da antocianina de uva, através da quantificação espectrofotomètrica de constituintes do sangue e medida de massa corporal de coelhos saudáveis. Rev Analytica 2004 12: 50-55. [ Links ]
22. Kontush A, Chapman MJ. Antiatherogenic small, dense HDL-guardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med 2006;3: 144-153. [ Links ]
23. Auger C, Caporiccio B, Landrault N, Teissedre PL, Laurent C, Cros GR, Besançon P, Rouanet J. Red wine phenolic compounds reduce plasma lipids and apolipoprotein B and prevent early aortic atherosclerosis in hypercholesterolemic golden Syrian hamsters (Mesocricetus auratus). J Nutr 2002; 132: 1207-1213. [ Links ]
24. Ho HM, Leung LK, Chan FL, Huang Y, Chen ZY. Soy leaf lowers the ratio of non-HDL to HDL cholesterol in hamsters. J Agrie Food Chem 2003; 51:4554-4558. [ Links ]
25. Macdonald IA, Mader JA, Bussard RG. The role of rutin and quercetin in stimulating flavonol glycosidase by cultured cell-free microbial preparation of human feces and saliva. Mutat Res 1983; 122: 95-102. [ Links ]
26. Silva RR, Oliveira TT, Nagern TJ, Pinto AS, Albino LFT, Almeida MR, Moraes GHK, Pinto JG. Efeito hipolipidêmico dos flavonóides naringina e rutina. ALAN 2001; 51: 258-264. [ Links ]
27. Rho KA, Kim MK. Effects of different grape formulations on antioxidative capacity, lipid peroxidation and oxidative DNA damage in aged rats. J Nutr Sci Vitaminol 2006; 52: 33-46. [ Links ]
28. Cook NC, Samman S. Flavonoids - Chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem 1996;7:66-76. [ Links ]
29. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95:351-358. [ Links ]
30. Reiter RJ. Oxidative processes and oxidative defense mechanism in the aging brain. FASEB J 1995; 9: 526-533. [ Links ]
31. Deschner EE. Dietary quercetin and rutin: Inhibitors of experimental colonic neoplasia. In Phenolic compounds in food and their effects on health II. Edited by Huang M, Ho C and Lee, Washington: C. ACS. 1992, pp.265-268. [ Links ]
32. Chen H, Li-jun L, Jian-jun Z, Bo X, Rui L. Effect of soybean oligosaccharides on blood lipid, glucose levels and antioxidant enzymes activity in high fat rats. Food Chem 2010; 119: 1633-1636. [ Links ]
33. Machado MRG. Bebida de soja fermentada com Lactobacillus acidophilus: viabilidade celular, avaliação sensorial, armazenamento e resposta funcional. Doctoral Thesis, College of Agronomy, Pelotas: Federal University of Pelotas, Brazil, 2007. [ Links ]
34. Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper: Bioquímica. 9o ed. São Paulo: Editora Atheneu. 2002. pp.276-278. [ Links ]
![]() Correspondence:
Correspondence:
Paula Ferreira de Araujo.
C/ Gomes Barbosa, 844, apartamento 402.
CEP: 36570-000 Viçosa. Minas Gerais. Brazil.
E-mail: paulaufpel@pop.com.br
Recibido: 22-IV-2010.
1a Revisión: 23-XI-2010.
Aceptado: 2-XII-2010.