Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.26 no.5 Madrid Set./Out. 2011
Enteral or parenteral nutrition in traumatic brain injury: a prospective randomised trial
Nutrición enteral o parenteral en lesión traumática cerebral: un estudio prospectivo y randomizado
C. Ma Justo Meirelles1 and J. E. de Aguilar-Nascimento2
1Health Science Postgraduate Course. Medical Sciences School. Federal University of Mato Grosso. Cuiaba. Brasil.
2Chairmain Professor of the Department of Surgery. Medical Sciences. School. Federal University of Mato Grosso. Cuiaba. Brasil.
ABSTRACT
Introduction: Few studies have evaluated the route of nutritional therapy in patients with head trauma.
Objective: We aimed at investigating whether early enteral (EN) or parenteral nutrition (TPN) may differ in protein/ calorie supply, serum glucose modifications, and acute phase response in patients with traumatic brain injury (TBI).
Methods: Twenty two patients with moderate TBI (Glasgow score between 9-12) were randomized to receive isocaloric and isonitrogeneous either EN (n = 12) or TPN (n = 10). The daily amount of calories and nitrogen (N) supplied, the nitrogen balance, and the daily serum level of glucose, C-reactive protein, and albumin were collected for 5 consecutive days. Clinical endpoints as length of stay and mortality were also compared.
Results: Mortality was 9.1% (two cases) with one case in each group. A progressive caloric deficit occurred in both groups (p = 0.001) without difference between them. The mean serum glucose level in TNP patients (134.4, 95% CI = 122.6 to 146.2 mg/dl) was significantly higher than in the EN group (102,4; 95% CI 91.6 to 113.2 mg/dL) (p < 0.001). There was a trend (p = 0.06) of 24 h urinary N loss to be greater in TPN group which received higher amounts of N than the NE group (p < 0.05). However, nitrogen balance was similar in the two groups. There was no difference in either the clinical outcome variables or the acute phase response.
Conclusion: Both routes were able to supply increasing provision of calories to brain injured patients. TPN provided significantly greater amount of nitrogen but losses were also greater. Nitrogen balance was similar with both types of therapy. Parenteral compared to enteral nutrition lead to greater hyperglycemia. There was no influence of the route in both the early inflammatory response and clinical outcome.
Key words: Enteral nutrition. Parenteral nutrition. Brain injury. Trauma. Nutritional therapy.
RESUMEN
Introducción: Pocos estudios han evaluado la ruta de la terapia nutricional en pacientes con traumatismo craneoencefálico.
Objetivo: El objetivo fue investigar si la nutrición enteral (EN) o parenteral (TPN) precoz puede ser diferente en suministro de calorías y proteinas, en las modificaciones de glucosa sérica, y en la respuesta de fase aguda en pacientes con lesión cerebral traumática (TBI).
Métodos: Veintidós pacientes con TBI moderado (puntuación de Glasgow entre 9 a 12) fueron aleatorizados para recibir de forma isocalórica y isonitrogenada EN (n = 12) o TPN (n = 10). La cantidad diaria de calorías y el nitrógeno (N) que se suministra, el balance de nitrógeno, y el nivel sérico de glucosa al día, la proteína C-reactiva, y la albúmina fueron recolectados durante 5 días consecutivos. Criterios de valoración clínicos como la duración de la estancia y la mortalidad también fueron comparados.
Resultados: La mortalidad fue del 9,1% (dos casos) con un caso en cada grupo. Un déficit calórico progresivo se produjo en ambos grupos (p = 0,001), sin diferencia entre ellos. El nivel de glucosa sérica media de los pacientes com TNP (134.4, IC 95% = 122,6 a 146,2 mg/dl) fue significativamente mayor que en el grupo com EN (102,4 IC 95%: 91,6 a 113,2 mg/dl) (p < 0,001). Se observó una tendencia (p = 0,06) en la pérdida urinaria de N em 24 h ser mayor en el grupo TPN que recibió mayor cantidad de N que el grupo EN (p < 0,05). Sin embargo, el balance de nitrógeno fue similar en ambos grupos. No hubo diferencia en cualquiera de las variables de resultado clínico o la respuesta de fase aguda.
Conclusión: Ambas rutas fueron capaces de suministrar cantidades diárias mayores de calorías para pacientes con lesión cerebral. Nutrición parenteral suministro cantidad mayores de nitrógeno, pero las pérdidas fueron tambiem mayores. El balance nitrogenado fue similar con ambos tipos de terapia. Parenteral en comparación con la nutrición enteral conduce a una mayor hiperglucemia. No hubo influencia de la ruta, tanto en la respuesta inflamatoria temprana y los resultados clínicos.
Palabras clave: Nutrición enteral. Nutrición parenteral. Lesión cerebral. Trauma. Terapia nutricional.
Introduction
The nutritional status of patients with traumatic brain injury (TBI) is a key factor to enhance morbidity and mortality. The lack of appropriate nutritional therapy (NT) leads to the onset of rapid malnutrition in such patients and may increase the risk for infections, muscle wasting, indications and maintenance of mechanical ventilation, and prolonged healing of wounds.1,2,3 Nutritional support in this subset of critically ill patients is a key component of the whole therapy.4,5,6
TBI patients experiment a hypermetabolic and hypercatabolic state with a severe loss of nitrogen and rapid deterioration of body lean mass.7,8 Resting energy expenditure in patients with isolated TBI showed is augmented with an average of 140% over a normal individual ranging from 120% to 250%, despite the use of sedation and muscular blockers.6,7,9
Approximately 40% of multiple trauma patients develop hospital malnutrition and are most prone to infection, increasing the mortality rate up to 60% in patients who stay in the ICU for over than five days.10 This may be attributed to systemic inflammation followed by immne paralysis which is frequently seen after trauma. In acute TBI, the activation of compensatory anti-inflammatory response syndrome (CARS) often leads to immunosuppression which may result in sepsis followed by multiorgan dysfunction syndrome and mortality.11
The hypermetabolism and hypercatabolism increase the loss of urinary nitrogen which can exceed 30 g of N per day. Thus NT is essencial in theses cases.12 However the route to deliver nutrients in TBI is still controversial. Comparisons between enteral nutrition (EN) and total parenteral nutrition (TPN) have consistently shown that feeding the patient via the gut leads to less infectious complications and reduces the length of stay.1,3 EN is more physiological and is the first option in critically ill patients because it maintains the integrity of the intestinal mucosal,1,3,13 prevents bacterial translocation, and improves the immunological response.14,15 However in a patient with severe TBI (GST < 8) there is an increased possibility of aspiration into the airways with enteral feeding. Morover the evolution of the prescription to meet the caloric goals is often most difficult with EN and thus may lead to undernutrition.1,3,14,16 On the other hand TPN is most associated with infectious complications, and is usually associated to hyperglicemia and overfeeding.17
A few studies have assessed TPN as opposed to EN in TBI. Thus we aimed at investigating the efficacy of EN or TPN to deliver nutrients in TBI patients. Secondly we compared the clinical outcome and the acute phase protein response with the two routes of nutritional therapy.
Methods
This was a clinical, prospective, randomised study. Inclusion criteria included patients of both sexes, aged between 18 and 60 years-old, admitted to the ICU of the Rondonopolis Regional Hospital (HRRoo), diagnosed with moderate TBI (Glasgow score 9-12) during the period August 2008 to June 2009. All the patients were included after confirmation of TBI performed by clinical, neurological assessment, and CT scan. The present study was approved by the Ethics Research Committee of the Júlio Müller University Hospital.
Exclusion criteria included: chronic renal failure (peritoneal or hemodialysis or creatinine > 2.5 mg/dl), history of chronic obstructive pulmonary disease, hepatic dysfunction or cirrhosis or bilirubin > 3 mg%; insulindependent diabetes mellitus; morbid obesity; preexisting malnutrition, defined as body mass index less than 17 kg/m2; pregnancy, immune depressive conditions, and associated abdominal trauma. Randomised patients were also excluded from either enteral or parenteral groups if for any reason they were not able to receive nutrition for more than two consecutive days.
Patients were randomised to receive either EN or NPT as soon as they were hemodinamically stable. Both groups were programmed to receive a 25 to 30 kcal/kg/day and 1.5 g/kg/day of protein. EN formula (Soya Diet; Support, Brazil (composition/100 mL: 3.6 g of protein [70% soy protein], 14 g of carbohydrate, and 3.5 g of lipids [77% long-chain and 23% median-chain fatty acids] added with casein to reach 1.5 g/kg/day) was administered via 8 or 10F oro- or naso-enteral feeding tube in gastric position with pump infusion.
TPN was administered by central venous access with the following composition/100 ml: 3.8 g of amino acids, 14 g of glucose and 3.3 g of lipids (50% longchain and 50% medium-chain fatty acids). Immunonutrients were not used. The required calories and protein for each individual in the two groups was set to be reached after three days of therapy.
Outcome variables
The clinical outcome variables compared were: mortality, morbidity, length of stay in ICU, and days of mechanical ventilation. In both groups the amount of calories and protein received daily was monitored. The difference between calories and protein prescribed and actually received was also calculated over the five days of follow up.
Patients were followed up in the first five days with daily blood samples for glucose, albumin, urea, creatinine, C-reactive protein (CRP) assays. The ratio CRP/albumin was defined as inflammatory index. A 24-h urine collection was conducted in all patients in each of the five days of the study. The urinary urea nitrogen (N) was assayed by automated colorimetric method. Nitrogen balance was calculated daily using the formula: nitrogen balance: N ingested-N excreted (24-hour urinary urea nitrogen x 0.46 + 2). The constant of 2 g of N in the nitrogen balance computation was an assumption of stool, integumentary, and other insensible nitrogen losses.
Sample size was calculated supposing a difference of 30 mg/dL with a standard deviation of 20 mg/dL in serum glucose levels. The sample size required to achieve 80% power and type 1 error of 5% was calculated to be 10 individuals per group. Data were analysed using the Statistical Package for Social Sciences (SPSS) version 10.0. The chi-square or Fisher exact test was used for comparisons between categorical variables. Repeated measures ANOVA test was used for independent and paired comparisons of continuous variables. A significance level of 5% was established.
Results
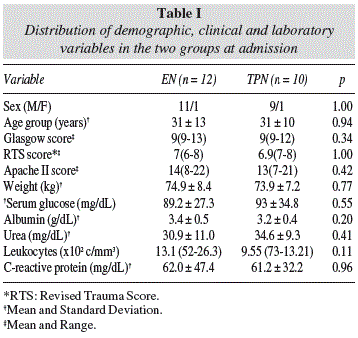
The distribution of demographic, clinical, and biochemical data at admission in the two study groups can be seen in table I. It was observed that the two groups were homogeneous in relation to the scores of severity assessment and biochemical tests performed at time of admission to hospital.
Clinical outcome
The mortality rate was 9.1% (2 cases) with one case in each group (EN = 8.3% and TPN = 10%; p = 1.00). There was no significant difference in morbidity between the two groups (p = 0.35). No cases of regurgitation in EN group were reported. There were four cases of complications (two cases of pneumonia and two cases of sepsis) in TPN group (40%) and two cases of pneumonia in the EN group (16.7%). The mean length of ICU stay was 14 days in both groups ranging from 5-26 days in EN group and from 6-24 days in TPN group (p = 0.86).
Nutritional outcome
Both groups received similar total amount of calories during five days (NE = 5,958 ± 3,619 kcal vs. TNP = 6,586 ± 1,052 kcal; p = 0.34). The amount of calories significantly increased (p < 0.01) in each consecutive day of the study in both groups (fig. 1). However, there was a progressive caloric deficit in the two groups (p = 0.001) without significant difference between who received either EN or TPN (p = 0.55).
Patients in both groups received increasing quantities of nitrogen day after day (p < 0.001). However, the group of patients treated with TPN received a significantly higher amount of N (p = 0.006) than the group treated with EN (fig. 2).
The urinary nitrogen assay showed that all the patients presented increased loss of nitrogen varying from 6.97 to 49.38 g of N/day. Although always negative, during the evolution of the nutritional therapy all patients presented a significant improvement (p = 0.001) in the nitrogen balance (fig. 3). The mean nitrogen balance was from -5.9 (95% CI: -7.9 to -4.0) g of N/day in the EN group, and -4.6 (95% CI: -6.7 to - 2.4) g of N/day in the TPN group (p = 0.34).
Figure 4 shows the total caloric and nitrogen intake, and the nitrogen loss during the five days of the study. There was no difference in the total of calories received by the two groups in the five days of the study. TPN group received more N but excreted more than the EN group.
Biochemical outcome
Serum glucose progressively increased in the two groups (p = 0.04), and was significantly higher in the group that received TPN (p < 0.01). Glicemia in the TPN group (134.4; CI 95% = 122.6-146.2 mg/dl) was significantly greater than the NE group (102.4; CI 95% = 91.6- 113.2 mg/dl) (p < 0.001). CRP increased and albumin decreased during evolution in all patients (fig. 5a and b). There was no difference in either the evolution or comparison of the two groups (p = 0.64) regarding the inflammatory index (CRP/albumin) (data not shown).
Discussion
The findings of this study showed that the two nutritional routes can offer increasing quantities of nitrogen and calories. However TPN have delivered nitrogen more efficiently. Other significant result was the confirmation that TBI patients lose increased amount of nitrogen independently of the chosen nutritional route. Nitrogen excretion in TBI patients may significantly increased for up to four weeks leading to enlarged nitrogen requirements18. In the present study the loss of nitrogen was so important that in both groups the daily provision of protein was unable to equilibrate the nitrogen balance. Nevertheless the early initiation of nutritional therapy by either NE or TPN routes was important to minimize the losses as reported earlier.19 In fact, early versus late initiation of nutritional therapy in TBI patients may improve the outcome. A recent systematic review made by Perel et al. found that early feeding may be associated with fewer infections and a trend towards better outcomes in terms of survival and disability.20
A few studies have investigated whether EN or TPN may influence the clinical outcome in TBI patients.8,19 The pooled results from trials analysed by a Cochrane systematic review suggested a trend towards better results with parenteral nutrition.20 Indeed, intolerance to enteral feeding in TBI may exist and impair the deliver of nutrients.21 However, we have not found any differences in clinical parameters in this study. Moreover, the final amount of both calories and proteins delivered in five days were not different between the groups.
The amount of nitrogen dispensed with TPN was greater than with EN. This is in agreement with other reports in critically ill22 and TBI patients.23 However, patients receiving TPN showed increased losses of nitrogen which in the end lead to similar nitrogen balance with the two therapies. We have not found similar result in the literature. One assumption for this interesting finding was that as the provision of intravenous nitrogen increases the excretion also augments. Further studies are necessary to explain these results.
Stress induces hyperglycaemia in various critical conditions such as TBI by a complex neuroendocrine response which includes enhanced gluconeogenesis, glycogenolysis, relative insulin deficiency, and impaired glucose utilisation.24 In these patients it has been shown that maintaining glycaemia near the normal levels may improve the outcome.25 In this context, TPN may promote poorer glucose control when compared to EN.22 Our findings are in accordance to the literature showing not only a trend to hyperglycemia after TBI7 but also that patients receiving TPN are most prone to present higher serum glucose levels when compared to those treated with EN.22
The organic response to trauma, especially after TBI is caracterized by an inflammatory response with the raise of serum IL-6 and positive acute-phase proteins and dropping of serum albumin and pre-albumin.26 The acute-phase proteins alterations were seen in the present study but they were not influenced by the type of nutrition provided. In the same way, our study failed to show differences in the clinical outcome. However, the sample was not calculated to achieve this endpoint and thus, our findings has to be analyzed with caution.
Another issue often claimed against EN in TBI is the possibility of pneumonia especially in those under mechanical ventilation.27 Although only a few cases were studied there was no case of frank regurgitation in patients underwent EN and the rate of pneumonia was similar in both groups. It is plausible to support the concept that the high incidence of pneumonia frequently seen in patients with head trauma is multifactorial, and not due to enteral feeding which may be protective as recently reported in one study.28
Conclusions
The overall results obtained by the present study allow us to conclude that both routes provide increasing and similar quantities of calories in TBI patients. TPN provided significantly more nitrogen than EN though the nitrogen balance is improved by both therapies. Hyperglycaemia is most prone to occur with TPN but the inflammatory response and clinical outcome were not affected by the type of nutrition provided.
References
1. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G; A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guideline for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2009; 33 (3): 277-316. [ Links ]
2. Vizzini A, Aranda-Michel J. Nutritional support in head injury. Nutrition 2010, Jun 23. [Epub ahead of print] [ Links ].
3. Singer P, Benger MM, Van den Berghe G et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr 2009;28 (4): 387-400. [ Links ]
4. Pepe JL, Barba CA. The metabolic response to acute traumatic brain injury and implications for nutritional support. J Head Trauma Rehabil 1999; 14: 462-474. [ Links ]
5. Krakau K, Omne-Ponten M, Karlsson T, Borg J. Metabolism and nutrition in patients with moderate and severe traumatic brain injury. A systematic review. Brain Inj 2006; 20: 345-367. [ Links ]
6. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. XII. Nutrition. J Neurotrauma 2007; 24 (Suppl. 1): S77-S82. [ Links ]
7. Raurich JM, Ibanez J. Metabolic rate in severe head trauma. JPEN J Parenter Enteral Nutr 1994; 18: 521-524. [ Links ]
8. Young B, Ott L, Haack D et. al. Effect of total parenteral nutrition upon intracranial pressure in severe head injury. J Neurosurg 1987; 67(1): 76-80. [ Links ]
9. Bratton SL, Chestnut RM, Ghajar J et al. XII. Nutrition. J Neurotrauma 2007; 24 (1): 77-82. [ Links ]
10. Goiburu ME, Goiburu MM, Bianco H, Díaz JR, Alderete F, Palacios MC, Cabral V, Escobar D, López R, Waitzberg DL. The impact of malnutrition on morbidity, mortality and length of hospital stay in trauma patients. Nutr Hosp 2006; 21 (5): 604-10. [ Links ]
11. Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci 2009; 14: 3795-813. [ Links ]
12. Härtl R, Gerber LM, Quanhong N. Effect of early nutrition on deaths due to severe traumatic brain injury. J Neurosurg 2008; 109:50-56. [ Links ]
13. Hadfield RJ, Sinclair DG, Houldsworth, PE et al. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med 1995;152: 1545-8. [ Links ]
14. Dock-Nascimento DB, Tavares VM, de Aguilar-Nascimento JE. Evolution of nutritional therapy prescription in critically ill patients. Nutr Hosp 2005; 20 (5): 343-7. [ Links ]
15. Niinikoski H, Stoll B, Guan X et al. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr 2004; 134: 1467-74. [ Links ]
16. Wernerman J. Paradigm of early parenteral nutrition support in combination with insufficient enteral nutrition. Curr Opin Clin Nutr Metab Care 2008; 11: 160-163. [ Links ]
17. Gulín Dávila J, López García VM. Implementation of the standards for monitoring parenteral nutritional support in the adult patient. Nutr Hosp 2010; 25 (3): 443-8. [ Links ]
18. Clifton GL, Robertson CS, Grossman RG, Hodge S, Foltz R, Garza C. The metabolic response to severe head injury. J Neurosurgery 1984; 60: 687-96. [ Links ]
19. Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, Borzotta R. Enteral versus parenteral nutrition after severe closed head injury. J Trauma 1994; 37:459-68. [ Links ]
20. Perel P, Yanagawa T, Bunn F, Roberts I, Wentz R, Pierro A. Nutritional support for head-injured patients. Cochrane Database Syst Rev 2006; (4): CD001530. [ Links ]
21. Norton JA, Ott LG, McClain C et al. Intolerance to enteral feeding in the brain-injured patient. J Neurosurg 1988; 68 (1); 62-6. [ Links ]
22. Gramlich L, Kichian K, Pinilla J et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition 2004; 20: 843-848. [ Links ]
23. Nataloni S, Gentili P, Marini B, Guidi A, Marconi P, Busco F, Pelaia P. Nutritional assessment in head injured patients through the study of rapid turnover visceral proteins. Clin Nutr 1999; 18 (4): 247-51. [ Links ]
24. Collier B, Dossett LA, May AK et al. Glucose control and the inflammatory response. Nutr Clin Pract 2008; 23: 3-15. [ Links ]
25. Finney SJ, Zekveld C, Elia A et al. Glucose control and mortality in critically ill patients. JAMA 2003; 290: 2041-2047. [ Links ]
26. Woiciechowsky C, Schöning B, Cobanov J, Lanksch WR, Volk HD, Döcke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma 2002; 52 (2): 339-45. [ Links ]
27. Acosta Escribano JA, Carrasco Moreno R, Fernández Vivas M, Navarro Polo JN, Más Serrano P, Sanchez Payá J, Caturla Such IJ. Gastric enteral intolerance in mechanically ventilated patients with traumatic cerebral lesion. Nutr Hosp 2001; 16 (6): 262-7. [ Links ]
28. Lepelletier D, Roquilly A, Demeure dit latte D, Mahe PJ, Loutrel O, Champin P, Corvee S, Naux E, Pinaud M, Lejus C, Asehnoune K. Retrospective analysis of the risk factors and pathogens associated with early-onset ventilator-associated pneumonia in surgical-ICU head-trauma patients. J Neurosurg Anesthesiol 2010; 22 (1): 32-7. [ Links ]
![]() Correspondence:
Correspondence:
Jose Eduardo de Aguilar-Nascimento.
Hospital Universitario Julio Muller.
Rua Estevão de Mendonça, 81, apto. 801.
78043-300 Cuiaba. Mato Grosso. Brazil.
E-mail: aguilar@terra.com.br
Recibido: 13-XI-2010.
Aceptado: 4-III-2011.