Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.26 no.6 Madrid Nov./Dez. 2011
Animal model of undernutrition for the evaluation of drug pharmacokinetics
Modelo de desnutrición animal para la evaluación de estudios de farmacocinética
M. Merino-Sanjuán1,2, A. Catalán-Latorre1,2, A. Nácher1,2, S. Miralles-Arnau1,2 and N. V. Jiménez-Torres1,3
1Department of Pharmacy Technology. Faculty of Pharmacy. University of Valencia. Valencia. Spain.
2Institute of Molecular Recognition and Technological Development (IDM). Polithecnic University. University of Valencia. Valencia. Spain.
3Pharmacy Service. Dr. Peset University Hospital. Valencia. Spain.
ABSTRACT
Background: Protein energy malnutrition is a public health problem affecting a great number of people. Pathophysiological imbalances in malnourished individuals have a profound impact on drug pharmacokinetics.
Objective: To develop an animal model of undernutrition using male Wistar rats to be used to assess, in further studies, the impact of nutritional status on the oral bioavailability and pharmacokinetics of drugs.
Desing: Animals were randomly assigned to one of two groups and fed different diets for 26 days: WN (well-nourished/regular diet, N = 61) and UN (under-nourished/protein-calorie restricted diet, N = 72). Assessment of the animals' nutritional status was performed taking into account serum albumin, total cholesterol level and total body weight. A kinetic model incorporating population kinetic analysis (NONMEM) was developed to analyze body weight versus time profiles in the adaptation period following administration of the two aforementioned diets.
Results: Serum albumin plasma levels were lower than 2.3 g/dL in 80% (60/72) of malnourished animals at the end of the adaptation period. The range of the total serum cholesterol was similar in both groups at the end of the adaptation period. Total body weight in all cases was less than 230 g for malnourished animals and higher than 240 g for well-nourished animals.
The kinetic model assayed was confirmed to be an expansion module characterized by linear weight gain and a decline module characterized by exponential weight loss, where the weight loss rate constant is an exponential function of time. The bootstrap resampling method confirmed the stability of the model eventually selected.
Conclusions: The animal model developed in this study is reliable and could be of use in evaluating the impact of nutritional state on the pharmacokinetics of drugs. The proposed mathematical model allows the body weight of animals to be predicted at a given time taking into account the diet followed in the experimental period.
Key words: Undernutrition. Rat. Animal model. Coding.
RESUMEN
Antecedentes: La desnutrición calórico protéica es un problema de salud que afecta a un número de personas muy elevado. Las alteraciones fisiopatológicos originadas por la desnutrición tienen una repercusión elevada en el comportamiento farmacocinético de los fármacos.
Objetivo: Desarrollar un modelo de desnutrición en ratas Wistar macho que pueda utilizarse para evaluar el impacto del estado nutricional sobre la biodisponibilidad oral y farmacocinética de los fármacos.
Diseño: Los animales fueron asignados de forma aleatoria a uno de los dos grupos y alimentados con diferentes dietas durante 26 días: WN (dieta normo-nutrida/regular, N = 61) y UN (dieta restringida/calórico-proteica N = 72). La clasificación del estado nutricional de los animales se realizó teniendo en cuenta los niveles de albúmina sérica y colesterol total, y el peso corporal total. Se ha desarrollado un modelo cinético poblacional para analizar la evolución del peso corporal de los animales durante el periodo de adaptación.
Resultados: Al final del periodo de adaptación los niveles de albúmina plasmática fueron inferiores a 2,3 g/dL en el 80% (60/72) de los animales desnutridos. Sin embargo, los valores de colesterol total en suero fueron similares en ambos grupos. El peso corporal total de los animales desnutridos fue inferior a 230 g y superior a 240 g para los animales alimentados en condiciones de normonutrición.
El modelo de cinético de evolución del peso seleccionado se caracteriza por una cinética combinada lineal y exponencial. La fracción lineal representa el aumento de peso, y la fracción exponencial, que a su vez es función del tiempo, caracteriza la pérdida de peso del animal. La estabilidad del modelo seleccionado se ha realizado utilizando la técnica de remuestreo (bootstrap) y la validación mediante el test de predicción visual (visual predictive check, VPC).
Conclusiones: El modelo animal desarrollado en este estudio puede ser de utilidad para evaluar el impacto del estado nutricional sobre la farmacocinética de los fármacos. El modelo matemático propuesto permite predecir el peso corporal de los animales, teniendo en cuenta la dieta la dieta administrada durante el período experimental.
Palabras clave: Desnutrición. Rata. Modelo animal. Codificación.
Introduction
Protein energy malnutrition is a public health problem affecting a great number of individuals. In the last three decades the prevalence of malnutrition in hospitalized patients has varied between 19 and 80% of patients according to the country and patient group in question.1
Pathophysiological derangements have a profound impact on drug disposition in malnourished patients, as they affect absorption, protein binding, hepatic metabolism and renal elimination. In fact, malnutrition has dramatic effects on small intestinal mucosal structure and transport activity, and its consequences for the absorption of nutrients and drugs are highly variable and contradictory.2 It can increase or decrease absorption, both in terms of rate (absorption rate constant) and extent (area under the plasma concentration time curve).3 Moreover, metabolic pathways can be impaired in a state of malnutrition; therefore, the major effects of malnutrition on drug disposition are a reduction in clearance and an increase in elimination half-life, both of which alter oral bioavailability.2 As a result, the poor prognosis of some patients could be related with the detrimental impact of malnutrition on the efficacy, toxicity and pharmacokinetics of the medication prescribed. 4-6 However, there are few studies which have evaluated the influence of malnutrition on the pharmacokinetics of drugs in under-nourished patients, probably due to the difficulty of carrying out these studies in this population.
The aim of the present study was to develop an undernutrition model using male Wistar rats as an animal model with which to assess, in further studies, the impact of nutritional status on the oral bioavailability and pharmacokinetics of drugs used to treat diseases associated with malnutrition, such as cancer, AIDS or Alzheimer's disease.
Material and methods
Legal and ethical requirements of the study
Male Wistar® rats were provided by the animal facility of the Faculty of Pharmacy, University of Valencia. All the assays described in the present work adhered to the Principles of Animal Care and were approved by the Faculty of Pharmacy Ethics Commission (Valencia, Spain).
Inclusion and exclusion criteria
The biometric and biochemical parameters used to include or exclude the animals in this study were sex, age, weight, serum albumin and total serum cholesterol.
A total of 133 male Wistar rats of 8-9 weeks of age, weighing 220-250 g, with a serum albumin ranging from 2.3 to 4.3 g/dL and total serum cholesterol ranging from 30 to 195 mg/dL, were included in this study. The animals were placed in individual polyethylene cages (22 x 22 x 16 cm) in a controlled room (22-23o C, 50-60% humidity) in a 12-hour light/dark cycle.
Maintenance of the animals
Animals were randomly assigned to one of two groups: WN (well-nourished/regular diet, N = 61) and UN (under-nourished/protein-calorie restricted diet, N = 72). Both groups were allowed free access to water, but food intake was controlled. The WN group was subjected to a standard pellet diet (2014 from Harlan laboratory) that fulfilled the normal daily requirements of a rat (14% proteins, dose of diet intake per day: 20 g/60.2 kcal) during an adaptation period of 26 days. The UN group received a diet that was altered in protein, carbohydrate and fat content (TD 99,168 from Harlan laboratory; 5% proteins, dose of diet intake per day: 10 g/38 kcal) for the same period of time.
Monitoring of animals
All the animals in both groups (WN and UN) were weighed daily, and serum albumin and total serum total cholesterol were quantified once a week (days 1, 7, 14, 21 and 26). For this purpose, a sample of blood was extracted by puncturing the saphenous vein of the hind limb and was then injected into Eppendorf® tubes heparinized 5%. The blood samples were centrifuged at 3,500 rpm for 10 minutes, and plasma was then collected. Serum albumin and total serum cholesterol were analyzed using standard commercial kits, according to the manufacturer's instructions (QCA Laboratory, Spain).
Assessment of the animals' nutritional status was carried out using serum albumin and total serum cholesterol levels as biochemical parameters and weight as a biometrical parameter. Serum albumin is an indicator of protein reserves and cholesterol is a caloric depletion parameter.7 Weight loss is also used to classify patients as malnourished.8
Animals were considered undernourished when their serum albumin level was under 2.3 g/dL and their body weight was under 240 g. Both parameters were used to classify the animals according to three different degrees of undernutrition: mild, moderate and severe. Scores were assigned by the authors based on CONUT, a screening tool for controlling nutritional status in hospitalized patients.7 Parameter values and the scores assigned according to degree of undernutrition are shown in table I.
In addition, body weight was used as biometrical parameter for growth modeling in both groups of animals (WN and UN).
Intestinal permeability
Intestinal injury was evaluated in 12 animals (6 wellnourished and 6 undernourished) by quantifying intestinal permeability using the lactulose/manitol (L/M) test, as previously described.9 Following overnight fasting, 2 mL of an oral solution containing 60 mg lactulose and 30 mg mannitol were administered by gastric tube feeding. Urine samples were collected for 5 hours using metabolic cages and were stored at -40o C for further analysis. Urinary lactulose and mannitol were measured using high performance liquid chromatography (HPLC).10 Results were expressed as a ratio of the percentage of the administered dose of the two molecules excreted in urine.
Kinetic calculations and statistical analysis
Kinetic models to evaluate the change in body weight during the adaptation period were simultaneously fitted to the data for 133 animals (61 well-nourished and 72 malnourished) using the NONMEM 6.0 program (Beal and Sheiner, 1992). The first-order (FO) method was used in the first estimation step. For all models, subroutine ADVAN6 and differential equations were used. The basic model being assayed was characterized by the following expression:

where W is animal body weight (g), kG is the weight gain rate constant (g/day) and kL is the weight loss rate constant (day-1). Equation 2 was also considered, in which the kL changes over time according to an exponential function, with kIL representing the moment at which the controlled feeding started

In each model the parameters (kG, kL, kIL and SLO) were considered as equals or different for the two groups of animals (a total of 12 models were assayed: 4 for equation 1 and 8 for equation 2).
The POSTHOC function of NONMEM enabled estimation of the individual kinetic parameters using a Bayesian approach, taking both individual observations and population effects into account. Both interindividual variability of kinetic parameters and residual error with respect to body weight were modelled as exponential according to equations 3 and 4:
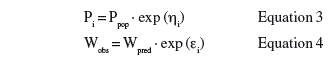
where Ppop is the population parameter value, h is a normally distributed zero-mean variable with the standard deviation w, Wobs is the observed weight, Wpred is predicted weight by individual parameter Pi, and e is normally distributed random variables with zero-mean and standard deviation s.
For the hierarchical model, improvement in the fit was assessed firstly according to the change in the minimum value objective function (MVOF). A change in MVOF of ≥ 6.63 was required for the addition of one fixed effect parameter to reach a statistical significance of p = 0.01. For the deletion of one fixed effect parameter from the full model, a change in MVOF of ≥ 7.88 was required to reach a statistical significance of p = 0.05. In addition, improvement in the fit (adding a parameter to or deleting it from the model) was determined by the change in the between subject and residual variability. The following were used as indicators of the goodness-of-fit in each of the models evaluated: the scatter plots of the correlation between individual- and population-predicted versus -observed body weight; weighted residual against weight; and weighted residual against time.
The bootstrap resampling technique was employed as an internal method to validate the final model. From the original data set, 1,000 replicates were generated by random sampling with replacement, and the final population kinetic model was fitted repeatedly to each replicate using the bootstrap option of Wings for NONMEM VI package (N Holford, version 222, May 2001, Auckland, New Zealand). The mean and median parameter estimates and their 95% confidence interval were obtained from the bootstrap replicates and compared with the population kinetic parameters from the original set of data. In addition, the selected model was evaluated by means of the visual predictive check. For this purpose, simulations of animal body weight-time profiles from 200 simulated populations were performed using the selected model, and each model parameter was estimated including between subject variability. For each group (well- and malnourished animals), the body weight-time profiles of the 5th, 50th and 95th percentiles were represented together with the corresponding observations.11
Statistical analysis of the results was carried out using the SPSS 15.0 and S.Plus 6.1 program version, and statistical significance was fixed at 0.05.
Results
Biochemical and biometric parameters
Figure 1 shows the density plots for serum albumin, total serum cholesterol and body weight (days 1 and 26) of WN and UN animals. Mean values, standard deviation and 95% confidence interval of these parameters (days 1 and 26) for both groups of animals are shown in table II. Statistically significant differences in serum albumin and body weight (p < 0.05) between WN and UN animals were detected from week 3 until the last day of the adaptation period. The range of the total serum cholesterol in both groups was similar at the end of the adaptation period (fig. 1) and statistical differences were obtained only at the end of the experience (day 26) (table II).
Undernutrition assessment and classification
Serum albumin levels at the end of the adaptation period (day 26) were lower than 2.3 g/dL in 80% (60/72) of the undernourished animals and in 11 % (7/61) of the well-nourished animals. Total body weight in all cases was less than 230 g for malnourished animals and higher than 240 g for well-nourished animals. On applying the criteria shown in table I, all malnourished animals were classified as undernourished: mild undernutrition status 18.1% (13/72), moderate undernutrition status 58.3% (42/72) and severe undernutrition status 23.6% (17/72).
Modeling animal body weight
Figure 2 shows individual animal body weight versus time profiles of well- and malnourished animals in the course of the adaptation period, during which weight gain in the well-nourished animals obeyed linear kinetics. However, a reduction of body weight was observed until day 15 of this period in malnourished animals, after which it stabilized. At the end of the adaptation period (26 days) the weight gain in the animals fed the standard diet (well-nourished) represented 19 % of their initial weight. However, during this period, the animals fed with the hypoproteic and hypocaloric diet (malnourished) reached a mean weight that represented 75% of the well-nourished group's final weight. Differences were statistically significant (p < 0.05). In the description of data, entering a parameter for well- or malnourished animals (e.g. weight gain/loss rate constants) offered significant improvement over assuming equal values of the parameters for both groups (equation 1: MOF = 8,256 considering the same parameter value for both groups and MOF = 6,648 when these values were different; and for equation 2: MOF = 6,725 and 6,423 for equal or different parameter values). Assuming that the weight loss rate constant changed over the time (equation 2) and that the parameters were different for each group, the best model was that shown in table III. Results of the bootstrap resampling technique are also provided (table III). Mean values of the bootstrap procedure with successful minimization were similar to the population parameter estimates of the original data set. Furthermore, fixed and random estimates of the original data set fell within the 95% confidence interval obtained for the bootstrap replicates.12 Figure 3 shows the correlation between individual- and population-predicted versus -observed body weight, weighted residual against weight, and weighted residual against time for the selected model.
Intestinal permeability
The ratio of lactulose and mannitol (L/M) excreted in urine was 0.15 ± 0.02 and (0.03 ± 0.004) in malnourished and well-nourished animals, respectively. Statistical differences were detected (p < 0.01).
Discussion
Malnutrition has been associated with variations in drug absorption and drug disposition with respect to protein binding, hepatic metabolism and renal elimination. As a consequence, drug toxicity increases and the response to treatment is altered.13 However, few studies have evaluated the influence of malnutrition on the pharmacokinetics of drugs used by malnourished patients, probably due to the difficulty of carrying out these studies in this population group. Therefore, the aim of the present study was to develop an animal model of protein energy malnutrition employing Wistar rats which could be used in further studies to assess the impact of nutritional status on the pharmacokinetics of drugs used in diseases associated with malnutrition.
There are many reports in the literature describing a model of protein calorie malnutrition, but we have observed a high variability among these models, particularly in two aspects. The first is the age of the animals included in the studies, and the second is the time during which animals are fed with a low-protein diet or allowed partial (or total) restricted access to a standard diet. In relation to age, most studies analyze the influence of partial deprivation of protein in the diet during pre- or postnatal periods (starting after birth or weaning), but few continue to observe the animals into adulthood, as we did in our experimental model. On the other hand, the time during which animals must follow a low-protein diet varies depending on the malnutrition level to be achieved; for example, three days for mild malnutrition, two weeks for moderate malnutrition,14 or four weeks for a severe malnutrition state.15
In our study, we proposed a animal model of malnourishment in young adult Wistar rats (8-9 weeks days old) based on feeding the animals a 10 g/day lowprotein diet for 23-25 days.
Serum albumin and total serum cholesterol were analyzed to assess proteic and energy basal levels to ensure that all animals were in optimum nutritional conditions when they were selected for the study. These parameters were also used to evaluate the degree and type of undernourishment among the animals in the UN group at the end of the study. Serum albumin concentration is usually employed as a nutritional biomarker in humans, even though its long half-life (20 days) does not make it a highly sensitive marker for acute changes in nutritional status. The high variability of these parameters according to the animal species and maintenance conditions in question makes it necessary to determine their value in each studied population.16 Serum albumin and total serum cholesterol values were obtained in our experimental conditions in accordance with this notion (table I).
The use of total serum cholesterol as a measure of malnutrition is controversial. In fact, due to its high variability total serum cholesterol has also been identified as a non-specific marker of malnutrition.17,18 However, some authors indicate the usefulness of this parameter in both humans and laboratory animals as a marker of the existence of metabolic syndrome.19 In clinical practice it has been observed that the total serum cholesterol concentration is reduced in malnourished patients with renal or hepatic insufficiency and malabsorption syndrome, and so some authors employ this parameter to determine nutritional status.
In our study, we assessed whether or not the diets administered to the animals induced significant changes in the range of total serum cholesterol over the course of the assay. We can affirm that this parameter did not reflect the nutritional state of the animals.
Consequently, the degree of nutritional undernourishment was evaluated using a total score based on serum albumin level and body weight (table I). The former has twice the rating of the latter, as it provides more information as a biomarker of undernutrition. Taking into account this criterion, we observed that the percentage of our animals that reached a state of moderate or severe malnutrition was above 80%.
In our research, variation of body weight during the 4 week experimental period showed differences between the well- and malnourished groups. Body weight increased when a non restricted protein caloric diet was administered. In contrast, in malnourished rats body weight evolved in two phases: there was a linear weight loss during the first 2 weeks, and a stabilization of body weight over the last 2 weeks of the experiment, reflecting a mechanism of adaptation to dietary restriction in adult rats. Our results are in accordance with those of Felgines C et al., who reported that body weight in dietary restricted animals stabilized after two weeks. In contrast, they observed that body weight loss in restricted aged rats was linear during the experiment, with no stabilization over time.20
Several growth equations exist according to the different approaches of authors who have studied this matter, and practically all agree that the growth curve acquires a sigmoid form.21,22 This is the result of two opposite factors that explain the growth -namely the intrinsic tendency towards unlimited increase- and restraints imposed by environmental resistance and aging.23 Consequently, most of the current growth equations consist of two modules, expansion and decline, which encapsulate the positive and negative factors of growth, respectively. The mathematical model developed and supported by our experimental data also considers these two processes, represented by a linear weight gain and exponential weight loss.
The factor (FKG UN = 0.313) used to quantify the modification of weight gain rate constant in malnourished animals (KG UN = 0.613 g/day) represents 31% of the weight gain rate constant in well-nourished animals. The fact that undernourished animals had a higher initial weight loss rate constant (FKIL [UN] = 18.3) suggests that, in the first stage of the conditioning period, the decline took place in dietary restricted rats at a higher rate than in well-nourished rats, and that this weight loss stabilized after 10-12 days of experiment as a result of the mechanism of adaptation to dietary restriction.
The visual predictive check and graphical diagnostics were performed to globally evaluate the kinetic model and ensure that the variability in the data was accurately described. The model evaluation was completely satisfactory and its appropriateness can be corroborated.
Some authors have reported that fasting or malnutrition has dramatic effects on small intestinal mucosal structure and transport activity. Mucosal atrophy induced by malnutrition reduces the total intestinal absorption of nutrients, although nutrient absorption can be normalized to mucosal mass by several mechanisms, including increased transporter gene expression, electrochemical gradients and changes in the ratio of mature to immature cells.3
Intestinal mucosal injury can be assessed by measuring the permeability of the mucosal barrier to small or large solutes. Lactulose and mannitol have previously been used to assess intestinal mucosal permeability in burn and critically ill patients. Mannitol (M), a smaller sugar, passes through aqueous pores in the cell membranes, and lactulose (L), a larger molecule, is absorbed paracellularly through tight junctions. Increased absorption of lactulose can reflect mucosal leakiness, and decreased absorption of mannitol can reflect decreased functional absorptive area. This study demonstrates that the L/M ratio was significantly greater in undernourished animals (p < 0.05), which is in accordance with the mucosal atrophy and disruption of tight junction epithelial cells associated with malnutrition status.24
We have previously employed the undernutrition animal model proposed in this study to evaluate the role of nutritional status in the absorption and bioavailability of leucine as a nutrient marker in enteral diets.25 and to determine the influence of protein-energy undernutrition in the absorption process of saquinavir.26 In both cases, our results indicated that absorption increased in the malnourished state, slightly in the case of leucine and to a statistically significant level in that of saquinavir (p < 0.05). Those results indicate that malnutrition status is a risk factor for saquinavir overdosing and toxicity and emphasize the need for an appropriate animal model with which to obtain information that can help to improve safety when drugs are administered to undernourished patients.
Conclusions
In summary, the large number of subjects included in our study and the high homogeneity of the malnutrition state in terms of final weight and final levels of serum albumin, which ranged between 1.4 and 1.89 g/dL in over 82% of malnourished animals, indicate that the animal model we have developed is reliable and could be of great use in evaluating the impact of nutritional state on the pharmacokinetics of drugs. The mathematical model proposed to describe the evolution of body weight in rats allows this parameter to be predicted at a given period of time taking into account the diet administered during the experimental period.
References
1. Waitzberg DL, Ravacci GR, Raslan M. Hospital hyponutrition. Nutr Hosp 2011; 26: 254-64. [ Links ]
2. Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Pharmacol 2010; 66: 1025-235. [ Links ]
3. Ferraris RP, Carey HV. Intestinal transport during fasting and malnutrition. Annu Rev Nutr 2000; 20: 195-219. [ Links ]
4. Tannuri U, Carrazza FR, Iriya K. The effects of glutamine-supplemented diet on the intestinal mucosa of the malnourished growing rat. Revista Do Hospital Das Clínicas 2000; 55: 87-92. [ Links ]
5. D'Incalci M, Steward WP, Gescher AJ. Modulation of response to cancer chemotherapeutic agents by diet constituents: is the available evidence sufficiently robust for rational advice for patients? Cancer Treatment Reviews 2007; 33: 223-9. [ Links ]
6. González-Hernández I, Jung-Cook H, Sotelo A. Effect of malnutrition on the pharmacokinetics of cefuroxime axetil in young rats. Journal of Pharmacy & Pharmaceutical Sciences 2008; 11: 9-21. [ Links ]
7. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20: 38-45. [ Links ]
8. Makhija S, Baker J. The Subjective Global Assessment: a review of its use in clinical practice. Nutr Clin Pract 2008; 23: 405-9. [ Links ]
9. Sun J, Wang L, Shen J, Wang Z, Qian Y. Effect of propofol on mucous permeability and inflammatory mediators expression in the intestine following traumatic brain injury in rats. Cytokine 2007; 40: 151-6. [ Links ]
10. García Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, Guerra Pinto A, Carolina Carneiro Aguirre A, Paiva Martins F, Marcos Andrade Goulart E, Sales Da Cunha A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol 2008; 43: 842-8. [ Links ]
11. Karlsson M HN. A tutorial on visual predictive checks. In: Europe PAGi, ed., 2008. [ Links ]
12. Bonate PL. Nonlinear mixed effects models: Theory. In: Bonate PL, ed. Pharmacokinetic-pharmacodynamic modeling and simulation. New York: Springer, 2006: 251-64. [ Links ]
13. Murry DJ, Riva L, Poplack DG. Impact of nutrition on pharmacokinetics of anti-neoplastic agents. Int J Cancer Suppl 1998; 11: 48-51. [ Links ]
14. Andreoli MF, Scalerandi MV, Borel IM, Bernal CA. Effects of CLA at different dietary fat levels on the nutritional status of rats during protein repletion. Nutrition 2007; 23: 827-35. [ Links ]
15. Kim YG, Kim SK, Kwon JW, Park OJ, Kim SG, Kim YC, Lee MG. Effects of cysteine on amino acid concentrations and transsulfuration enzyme activities in rat liver with protein-calorie malnutrition. Life Sci 2003; 72: 1171-81. [ Links ]
16. J. M. Zúñiga JAT, S. N. Milocco, R. Piñeiro. Ciencia y tecnología en protección y experimentación animal. McGraw-Hil/Interamericana de España, 2001. [ Links ]
17. López Martínez J, Sánchez Castilla M, Ordonez González FJ, Temprano Vázquez S, García de Lorenzo A, del Nogal Saez F. The usefulness of cholesterol as a nutritional-metabolic marker in the septic patient. Nutr Hosp 1995; 10: 24-31. [ Links ]
18. Mishra SK, Bastola SP, Jha B. Biochemical nutritional indicators in children with protein energy malnutrition attending Kanti Children Hospital, Kathmandu, Nepal. Kathmandu Univ Med J (KUMJ) 2009; 7: 129-34. [ Links ]
19. Cambri LT, Ghezzi AC, Ribeiro C, Dalia RA, de Mello MA. Recovery of rat growth and lipid profiles in adult rats subjected to fetal protein malnutrition with a fructose-rich diet. Nutr Res 2010; 30: 156-62. [ Links ]
20. Felgines C, Savanovitch C, Farges MC, Cynober L, Vasson MP. Protein metabolism in rats during long-term dietary restriction: influence of aging. JPEN J Parenter Enteral Nutr 1999; 23: 32-7. [ Links ]
21. Kaps M, Herring WO, Lamberson WR. Genetic and environmental parameters for traits derived from the Brody growth curve and their relationships with weaning weight in Angus cattle. J Anim Sci 2000; 78: 1436-42. [ Links ]
22. Berlanga García ME MAA, Luque Moya A.J. Growth curve in retinto beef cattle from birth to weaning. Arch Zootec 1995; 44: 179-92. [ Links ]
23. Zeide B. Analysis of growth equations. Forest Science 1993; 39: 26. [ Links ]
24. Tannuri U, Carrazza FR, Iriya K. The effects of glutamine-supplemented diet on the intestinal mucosa of the malnourished growing rat. Rev Hosp Clin Fac Med Sao Paulo 2000; 55: 87-92. [ Links ]
25. Miralles-Arnau S, Nacher A, Jimenez A, Jimenez-Torres NV, Merino-Sanjuan M. Impact of nutritional status on the oral bioavailability of leucine administered to rats as part of a standard enteral diet. Clin Nutr 2011. [ Links ]
26. Catalan-Latorre A, Nacher A, Merino V, Jimenez-Torres NV, Merino-Sanjuan M. In Situ Study of the Effect of Naringin, Talinolol and Protein-Energy Undernutrition on Intestinal Absorption of Saquinavir in Rats. Basic Clin Pharmacol Toxicol 2011. [ Links ]
![]() Correspondence:
Correspondence:
Matilde Merino-Sanjuán.
Department of Pharmacy and Pharmaceutical Technology.
Faculty of Pharmacy.
University of Valencia.
Vicente Andrés Estellés avenue.
46100 Burjassot, Valencia (Spain).
E-mail: Matilde.Merino@uv.es
Recibido: 18-VII-2011.
Aceptado: 20-VII-2011.
















