Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.26 no.6 Madrid nov./dic. 2011
Effectiveness of perioperative glutamine in parenteral nutrition in patients at risk of moderate to severe malnutrition
Efectividad de la utilización perioperatoria de glutamina en nutrición parenteral en pacientes con riesgo de desnutrición moderada-severa
G. Mercadal Orfila1 and J. M. Llop Talaverón2
1Department of Pharmacy. Hospital Mateu Orfila. Maó. Menorca. Spain.
2Servicio de Farmacia. Hospital Universitari de Bellvitge. Hospitalet de Llobregat. Barcelona. Spain.
ABSTRACT
Background & aims: To determine whether perioperative glutamine supplementation of parenteral nutrition (PN) has an impact on morbidity and mortality of patients with previous moderate to severe risk of malnutrition.
Methods: A quasi-experimental study was conducted comparing the following groups: Control group 1: without glutamine dipeptide supplementation of PN during the perioperative period. Control group 2: PN was supplemented with glutamine dipeptide (0.4 g/kg/day) after surgery only. Group 3 (follow-up group): PN was supplemented with glutamine dipeptide (0.4 g/kg/day) in the perioperative period. Postoperative morbidity and mortality was recorded.
Results: Sixty-seven patients matched for baseline and surgical characteristics were recruited into the study. Univariate analysis showed a lower incidence of hyperglycemia and ICU admission in group 3, and a trend to significance (P = 0.078) in terms of a lower incidence of infection. In the multivariate analysis, only group 3 met the models of ICU admission (OR = 0.28), hyperglycemia (OR = 0.11), and renal failure (OR = 0.19).
Conclusions: The results show that perioperative use of glutamine dipeptide in patients at risk of moderate to severe malnutrition before surgery is an effective option for decreasing the morbidity associated with malnutrition, as it improves blood glucose modulation and reduces infection and ICU stay.
Key words: Glutamine. Perioperative. Surgery. Malnutrition. Parenteral nutrition.
RESUMEN
Introducción y objetivos: Determinar si la aditivación de glutamina perioperatoriamente en Nutrición parenteral (NP) influye sobre la morbimortalidad en pacientes con riesgo previo de desnutrición moderada-severa.
Métodos: Se realizó un estudio cuasiexperimental en el que se comparaban: Grup control 1: sin suplemento de glutamina en NP en el perioperatorio. Grup control 2: sólo postcirugía se les suplementó con glutamina (0,4 g/kg/día) la NP. Grupo 3 seguimiento (prospectivo): perioperatoriamente suplemento de glutamina (0,4 g/kg/día) en la NP. Se registró la morbimortalidad postoperatoria.
Resultados: Se reclutaron 67 pacientes homogéneos en cuanto a características de base y quirúrgicas. El análisis univariante mostró menor incidencia de hiperglucemia y estancia en UCI del grupo 3, y tendencia a la significación (p = 0,078) en cuanto a la menor incidencia de infección. En el análisis multivariable, sólo el grupo 3 entró en los modelos de estancia en UCI (OR = 0,28), hiperglucemia (OR = 0,11) y fracaso renal (OR = 0,19).
Conclusiones: Los resultados obtenidos indican que el uso de glutamina a nivel perioperatorio en pacientes con desnutrición moderada-severa, previa a la cirugía, es una opción eficiente en la reducción de la morbilidad asociada a la desnutrición en términos de mejorar la modulación glucémica, reducción de la infección y de la estancia en UCI.
Palabras clave: Glutamina. Perioperatorio. Cirugía. Malnutrición. Nutrición parenteral.
Introduction
There is ample evidence suggesting that malnutrition is among the most significant clinical problems in hospitalized patients.1 It has been shown to intrinsically increase the complications of the disease causing admission and its related conditions. Malnutrition also increases the risk of infection, impairs response to treatment, and decreases the degree of immune response. The effects associated to a longer hospital stay and the costs derived from the abovementioned complications and prolongation of hospital stay are also potential consequences of malnutrition.2
In Spanish hospitals, the prevalence of malnutrition among surgical patients ranges from 13%-48.1%.3 In addition, the nutritional status worsens in 25% of these patients during hospital admission. Such impairment is related to the condition leading to admission, the fasting period required by the surgical procedure performed, particularly in gastrointestinal surgery, and the potential postoperative complications causing a protracted catabolic state.
In this setting, perioperative nutrition in elective surgery represents a therapeutic option to achieve a positive nitrogen and calorie balance, providing patients and adequate nutritional support to cope with surgical stress and the postoperative catabolic period.
Both the ASPEN (American Society of Parenteral and Enteral Nutrition)4 and the ESPEN (European Society of Parenteral and Enteral Nutrition)5 guidelines establish the need for nutritional support with immunomodulators in patients undergoing gastrointestinal tract surgery who have moderate to severe malnutrition. Among these immunomodulators, emphasis is placed on use of glutamine dipeptides.6
Various metaanalyses7,8,9,10 have demonstrated that use of glutamine in critically ill, surgical patients is associated to a positive nitrogen balance, a decreased infection rate, shorter hospital stay, and a lower mortality in critically ill patients in whom high glutamine doses are used. However, the value of glutamine in parenteral nutrition, particularly as regards perioperative use in patients with moderate to severe malnutrition, has not been established yet.
The ESPEN guidelines5 state that surgical patients who are candidates to receive perioperative nutritional support are those at nutritional risk in the 10-14 days prior to surgery. This condition even warrants a delay in surgery in patients who are at great risk of severe malnutrition.
Patients and methods
A quasi-experimental study was conducted on patients of both sexes aged > 18 years undergoing gastrointestinal surgery and who were candidates to receive perioperative nutritional support, i.e. patients at moderate to severe nutritional risk (weight loss > 5%- 10% in the past 6 months, body mass index (BMI) < 18.5 kg/m2, subjective global assessment (SGA) B-C, serum albumin < 3.5 mg/dL, or prealbumin < 15 mg/dL (no evidence of kidney or liver disease)). Exclusion criteria included chronic renal failure requiring dialysis, acute renal failure not subject to hemofiltration (creatinine > 2.2 mg/dL), liver failure with encephalopathy, severe metabolic acidosis, pregnant or lactating women, participation in another study, or a psychiatric condition preventing understanding of the study by the patient.
Three cohorts were compared:
- Group 1 (control 1) (-/-): patients with no glutamine dipeptide supplementation of parenteral nutrition (PN) before or after surgery who were provided perioperative nutritional support according to nutritional needs and expected duration of fasting (period from March 2006 to March 2007).
- Group 2 (control 2) (-/G): patients received glutamine dipeptide supplementation (0.4 g/kg/day) of PN in the postoperative period only, providing perioperative nutritional support according to nutritional needs and expected duration of fasting (period from March 2007 to March 2008).
- Group 3 or follow-up group (G/G): patients received glutamine dipeptide supplementation (0.4 g/kg/day) of PN before and after surgery according to nutritional needs and expected duration of fasting (period from March 2008 to March 2009).
Nutritional support with PN was started in all groups 2-3 days before surgery.
Calorie provision was based on the protocol used at our hospital: Baseline energy expenditure was estimated using the Harris Benedict formula, multiplied by a stress factor (Long) and by the activity factor.
In the groups with no glutamine supplementation, 4 g/kg/day of glucose, 1 g/kg/day of lipids, and 1.5 g/kg/day of protein were administered, while groups with glutamine supplementation received 1.1 g/kg/day of protein plus 0.4 g/kg/day of glutamine.
The following variables were recorded:
Demographic variables: gender and age; anthropometric parameters: weight, height, BMI, SGA, weight loss in the past 6 months; postoperative clinical course: incidence of fistula (spontaneous or due to suture dehiscence); incidence of intestinal failure (retroperitoneal bleeding, abscess and/or peritonitis); incidence of renal failure (serum creatinine > 2.2 mg/dL or serum urea > 80 mg/dL), incidence of hyperglycemia (serum glucose > 160 mg/dL); days on PN, days at ICU; hospitalization days; respiratory tract, urinary tract, and wound infections, defined as the presence of a respiratory, urinary, or wound infectious sites plus two of the following: WBC count > 12,000 x 106/L; fever > 38 o C; heart rate > 90 beats per minute, or PCO2 < 31 mmHg; death during hospital admission; death at 6 months; chemistry and hematology tests the day before surgery and on days 3 and 7 after surgery, and on the last day of total parenteral nutrition (TPN).
Ethics
This study was approved by the Hospital ethics committee, and all patients were informed about the objectives, methods and signed a informed consent.
We didn't have any grant or funding for this study.
Statistical analysis
All data are given as means, standard deviations and percentages. SPSS Version 12 statistical software for Microsoft Windows (SPSS Inc., Chicago, IL, USA) was used for all analyses. A univariate analysis was first performed comparing the different quantitative and qualitative variables between the three study groups, and was followed by a multivariate analysis.
Biochemical and hematological variables were also analyzed and compared, first between groups and then within each group, using an analysis of variance (ANOVA) and a Wilcoxon signed ranks test for related samples respectively.
When variables were normally distributed, parametric tests were performed: a Chi-square test to compare categorical variables, a Student's t test for quantitative variables, and a paired t test for related samples.
When the Kolmogorov-Smirnov test showed a nonnormal distribution, non-parametric tests were used: a Kruskal-Wallis test for independent samples, and a Wilcoxon signed ranks test for two related samples. A value of P < 0.05 was considered statistically significant.
Backward stepwise logistic regression was used to explain the categorical variables recorded in the study through the predictor variables, to determine explanatory factors or variables influencing the responses under study.
Backward stepwise Cox regression was used to explain the quantitative variables recorded in the study through the predictor variables, to determine explanatory factors or variables influencing the responses under study.
Results
Sixty-seven patients who met the inclusion criteria were recruited into the study. Seventeen patients were not recruited into the study because they met some of the exclusion criteria.
The size of our hospital (140 beds) and the recruitment period are the reasons for the small sample size. However, the results achieved allowed us for evaluating and verifying the effectiveness of perioperative glutamine supplementation in surgical patients with prior moderate to severe malnutrition.
All three groups had a higher proportion of men.
Analysis of variance showed no statistically significant differences in age, income, gender, current weight, usual weight, height, weight loss and BMI between the groups, which were therefore matched in the anthropometric variables studied (table I). Similarly, no differences were seen between the groups in terms of nutritional assessment before surgery, as established by the SGA questionnaire. This suggested that patient groups were similar in terms of nutritional status, as assessed by the aforementioned structured questionnaire at the beginning of the study.
As regards the Kruskal-Wallis test, no differences were found in the supply of glucose, nitrogen, lipids, or kcal between the groups. The most common surgeries performed were hemicolectomy, small bowel resection, and gastrectomy.
Table II shows that the G/G group spent less days at the ICU and on PN, and although statistical significance was not reached in the Kruskal-Wallis test, days at the ICU almost showed statistical significance (P = 0.074). A lower incidence of hyperglycemia and a lower proportion of patients staying at the ICU (yes/no) were seen in the G/G group as compared to the other two groups.
With regard to the presence of infection (yes/no), a nearly significant (P = 0.078) lower incidence of infection was recorded with glutamine use.
Remarkably, the linear trend test showed a linear relationship between glutamine supplementation and wound infection (P = 0.05), hyperglycemia (P = 0.002), ICU stay (P = 0.03), and the presence of infection (yes/no) (P = 0.025).
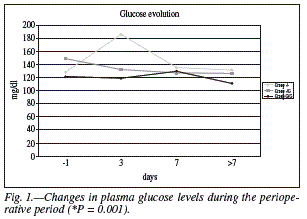
Results of the multivariate analysis (tables III and IV) showed the G/G group to be included in the models of ICU stay, hyperglycemia, and renal failure, but this did not occur with group -/G, thus suggesting a greater effectiveness of glutamine supplementation before and after surgery as compared to glutamine addition after surgery only or non-addition of glutamine before or after surgery. With regard to the biochemical and hematological tests, intergroup analysis showed significant differences on day 3 after surgery in glucose (Fig. 1) (-/-: 185.7 ± 86 vs. -/G: 132.074 ± 31.38 vs. G/G: 145.39 ± 62.9; P = 0.001) and albumin (-/-: 2.38 ± vs. 0.48 -/G: 2.71 ± 0.55 vs. G/G: 2.91 ± 0.57; P = 0.013), with groups -/G and G/G showing lower glucose values and higher albumin values than group -/-. This trend was also seen in the final TPN analysis for both glucose and albumin, although differences were not statistically significant.
In addition, blood testing at day 7 after surgery showed differences in WBC counts, with groups -/G and G/G reaching values within the reference range (4- 11 x109/L), while group -/- had mean values above the range (13.75 x109/L) suggesting leukocytosis.
Finally, intragroup results showed the G/G group to have the lowest reduction in blood albumin after surgery (albumin reduction in each group: -/-: = 0.95 g, -/G = 0.86 g, G/G = 0.7 g). In addition, only group -/- showed a significant increase in blood glucose from the preoperative analysis to day 3 after surgery (128.19 ± 40 vs. 185.7 ± 86.87; P = 0.003), with no significant increase in groups -/G (148.6 ± 200 vs. 132.07 ± 31; P > 0.05) or G/G (121.68 vs. ± 39.97 vs. 119.12 ± 44.72; P > 0.05).
Discussion
Few studies have reported the effect of perioperative intravenous glutamine supplementation in gastrointestinal surgery. Among studies using perioperative glutamine, some have measured analytical variables, while others have assessed morbidity and mortality. Among the former, Exner et al.12 showed perioperative glutamine administration to be associated with faster immune competence recovery, as measured by percent recovery of TNF-α secretion 48 hours after surgery. On the other hand, Yao et al.13 reported in 2005 a greater restoration of plasma endotoxin inactivation capacity (EIC) and a significant increase in CD4+ count on days 1 and 4 after surgery in the perioperative glutamine group as compared to the control group. Clinically, a reduction in hospital stay was seen in the intervention group (11.7 vs. ± 2 days vs. 10.6 ± 1.2; P = 0.03), but no differences were found in infectious complications.
In 2008, Yeh et al.14 also showed a reduction in hospital stay (16.3 days ± 21.3 vs. 12.2 ± 6.8 days; P = 0.299) and a decrease in postoperative albumin and Creactive protein levels and CD8+ percentage with lower values in the perioperative glutamine group as compared to the control group, thus suggesting attenuation of inflammation and immunosuppression as well as less nutritional depletion.
Fan15 reported improved maintenance of GSH levels, GSH/GSSG ratio, RBC counts and albumin levels, but there were no differences in the immunological indices recorded or in liver function parameters.
A study by Asprer16 where glutamine was only added before surgery showed increases in WBC, granulocyte, and lymphocyte counts which were not maintained after surgery when supplementation was discontinued.
Finally, the Gianotti study17 showed that in well nourished patients undergoing elective abdominal surgery, perioperative glutamine supplementation through glucose saline was not associated to a decreased morbidity and mortality when compared with the control group.
Our study had methodological differences with respect to the aforementioned publications because we used two control groups: one group with no glutamine addition before or after surgery, and a second control group in which glutamine was only added after surgery, thus allowing for more complete assessment of the effects of perioperative glutamine supplementation.
If our results are compared to those of the abovementioned studies on perioperative use of glutamine, our findings agree with those of Yeh and Yao13,14 as regards reduction of hospital stay, though the differences did not reach statistical significance.
As regards biochemical variables, it should be noted that, in agreement with Yeh,14 a lower decrease was seen in albumin levels at day 3 after surgery in the G/G group as compared to the control groups (0.9 g/ dl vs. 0.86 g/dl vs. 0.7 g/dl; P = 0.013). However, no differences were seen between the study groups in the evolution of C-reactive protein levels.
With regard to the Gianotti study,17 our findings suggest that in patients with a higher surgical risk, perioperative use of glutamine in TPN is associated with less postoperative morbidity. Our patients had a moderate to severe risk of malnutrition before surgery, with weight losses ranging from 8%-11%, while patients in the Gianotti study were well nourished, with a weight loss of 1.4%. Moreover, Gianotti used 5% dextrose as a vehicle for glutamine dipeptides, while total parenteral nutrition (TPN) was used in our more severely malnourished patients. Thus, our results suggest that therapeutic use of glutamine before gastrointestinal surgery would be warranted in patients with prior moderate to severe malnutrition. This patient subgroup is more similar to critical or trauma patients, in whom glutamine supplementation has been shown to decrease morbidity and mortality.7,18 This may be explained by a greater demand of glutamine, an increased glutamine metabolism and/or baseline deficits.17 Evidence for this is provided by the results of our study, showing improvements such as a decreased incidence of infection, an improved glucose metabolism, and a shorter ICU stay.
Special mention should be made of the role of glutamine in control of blood glucose homeostasis, as reflected by our data generated from both the univariate and multivariate analyses (tables II and IV; fig. 1).
We had already seen this result in a previous study by our group on glutamine supplementation in critically ill patients19 which showed the protective effect of glutamine (OR: 0.38, CI: 0.19-0.75) upon hyperglycemia. The capacity of glutamine to reduce insulin resistance in critically ill patients has also been confirmed by Dechelotte,18 who reported a lower incidence of hyperglycemia and less need for insulin in the group receiving glutamine supplementation. Mention should also be made to the study published by Bakalar,20 who found also a better blood glucose control in multiple trauma patients administered TPN supplemented with glutamine.
This effect of glutamine on glucose homeostasis is clearly important because of the poor prognosis of patients showing insulin resistance and persistent hyperglycemia, who have an increased incidence of infection and mortality.21 This mechanism could partly contribute to decrease infectious complications in patients in whom glutamine is added to TPN. In this regard, there are studies reporting an association between use of glutamine and protection against infection. At surgical level, 5 studies included in the metaanalysis conducted by Zheng10 (215 patients) reported infection, and pooled analyses showed a reduction in infectious episodes in the glutamine group as compared to the control group (OR = 0.24, 95% CI = 0.06, 0.93; P = 0.04). Dechelotte (18) also reported a lower incidence of nosocomial pneumonia in the glutamine versus the control group (17.24% vs. 33.9%; P < 0.05). Bonet22 in turn reported lower rates of nosocomial pneumonia (8.04% vs. 29.25%) and urinary tract infections (2.5% vs. 16.7%).
Wound, urinary tract, and respiratory tract infections reported in our study showed no statistically significant differences when considered separately. However, when these variables were combined as the variable "Presence of one or more infections (respiratory, urinary and/or pulmonary)" versus the groups showing no infection, no statistically significant differences were seen between the three study groups, but a trend to significance was seen (P = 0.078), and a linear relationship was found between glutamine supplementation and a lower infection rate (P = 0.025). As regards noninfectious postoperative complications, no statistically significant differences were found between the groups in the incidence of fistula or intestinal failure, but addition of glutamine before and after surgery was shown to protect against renal failure (OR = 0.19, 95% CI = 0.083-0.74). This result had already been found by our group in a study on use of glutamine in critically ill patients conducted in 2006.19
It should be noted that glutamine is the main substrate for ammonia synthesis in the kidney, and is therefore involved in regulation of acid-base balance. This is particularly important considering that renal failure is a clinical condition frequently associated to sepsis, trauma, and multiple organ failure in critically ill hospitalized patients.23
With regard to hospital stay, 6 studies included in the meta-analysis conducted by Zheng10 (291 patients) in which glutamine dipeptides were added to parenteral nutrition given to surgical patients showed a positive effect of glutamine, which shortened hospital stay (-3.55, 95% CI = -5.26, -1.84; P < 0.00001). This is consistent with the results of our study, where mean stay, ICU stay, and days on TPN were less in the groups given glutamine supplementation as compared to the group with no glutamine supplementation of parenteral nutrition; however, differences were not statistically significant (table II).
Moreover, logistic regression showed addition of G/G to be a protective factor, thus obviating the need for ICU care (OR = 0.289, 95% CI = 0.135-0.476) (table IV). This not only has clinical implications, but also economic effects, since a shorter hospital stay implies significant cost savings. Thus, Mertes24 estimated at $42,075 the savings associated to a mean reduction in hospital stay of 4.7 days in patients given glutamine supplementation. Among factors influencing a reduction in hospital stay as a result of glutamine use, mention should be made of accelerated healing, recovery of intestinal mucosal integrity, and prevention of potential infections through blood glucose regulation secondary to the effects of glutamine.
As regards mortality, the recent meta-analysis published in 2008 by Jones9 in critically ill patients including studies using enteral or parenteral glutamine supplementation revealed a significant reduction in mortality (RR 0.75, 95% CI = 0.59-0.96; P < 0.02). It may therefore be stated that there is a strong tendency to a beneficial effect of glutamine administration through parenteral nutrition in critically ill patients, especially those given TPN with glutamine for more than 10 days (25-27). In our study, mortality rates were similar in all three groups (19% vs. 14.8% vs. 15.8%). This agrees with the findings by Mertes24 and Jacobi28 in surgical patients in whom glutamine was administered by the parenteral route.
The above considerations suggest a greater impact of glutamine in terms mortality reduction in critically ill patients (who have a greater glutamine depletion) than in surgical patients. In addition, several survival studies have shown an increased survival in patients treated with glutamine. Thus, Griffiths'26 demonstrated longer survivals in critically ill patients when glutamine was added in TPN, with a resultant 50% reduction in hospital costs. Goeters29 in turn reported a greater 6-month survival rate in critically ill patients when administered TPN for more than 9 days.
Our study showed no differences in 6-month survival rates between the groups.
Finally, no significant changes were seen in renal or hepatic function parameters, which suggests that use of glutamine dipeptides in TPN is adequate, as shown by the multiple studies reporting its good tolerability and absence of side effects.30
Conclusion
Our results suggest that perioperative use of glutamine in patients with moderate to severe malnutrition before surgery is an effective option for reducing morbidity associated to malnutrition because it enhances blood glucose modulation, reduces infection, and shortens ICU stay.
Moreover, perioperative use of glutamine provides a greater effectiveness as compared not only to the lack of glutamine supplementation after surgery, but also to glutamine addition after surgery only, as is the usual clinical practice.
Acknowledgements
We thank the Departments of Surgery, Pharmacy and Biochemistry of Mateu Orfila Hospital for their cooperation in the present study.
We also thank Dr. Fernandez and Dr. Remesar of the Department of Nutrition and Bromatology (School of Biology-Universitat de Barcelona) for the technical advice provided.
References
1. De Ulibarri JI. Hospital malnutrition. Nutr Hosp 2003; 18 (2): 53-6. [ Links ]
2. Stratton RJ GC, Elia M. Disease-related malnutrition: an evidence-based approach to treatment: Oxon, UK: CABI Publishing; 2003. [ Links ]
3. Abdel-lah Mohamed A ÁHJ. Guia de actuación: Soporte nutricional en el paciente quirúrgico 2009. [ Links ]
4. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr 2002; 26 (1 Suppl.): 1SA-138SA. [ Links ]
5. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr 2009; 28 (4): 378-86. [ Links ]
6. Souba W. Nutritional support. N Engl J Med 1997; 336 (1): 41-8. [ Links ]
7. Novak F, Heyland DK, Avenell A, Drover JW, Su XY. Glutamine supplementation in serious illness: A systematic review of the evidence. Critical Care Medicine 2002; 30 (9): 2022-9. [ Links ]
8. Jiang Z-M JH, Fürst P. The impact of glutamine dipeptides on outcome of surgical patients: systematic review of randomized controlled trial from Europe and Asia. Clin Nutr 2004; S1: 17-23. [ Links ]
9. Jones NE, Heyland DK. Pharmaconutrition: a new emerging paradigm. Current Opinion in Gastroenterology 2008; 24 (2): 215-22. [ Links ]
10. Zheng YM, Li F, Zhang MM, Wu XT. Glutamine dipeptide for parenteral nutrition in abdominal surgery: A meta-analysis of randomized controlled trials. World Journal of Gastroenterology 2006; 12 (46): 7537-41. [ Links ]
11. Ikeda K, Kimura Y, Iwaya T, Aoki K, Otsuka K, Nitta H et al. Perioperative nutrition for gastrointestinal surgery. Nippon Geka Gakkai Zasshi 2004; 105 (2): 218-22. [ Links ]
12. Exner R, Tamandl D, Goetzinger P, Mittlboeck M, Fuegger R, Sautner T et al. Perioperative GLY-GLN infusion diminishes the surgery-induced period of immunosuppression-Accelerated restoration of the lipopolysaccharide-stimulated tumor necrosis factor-alpha response. Annals of Surgery 2003; 237 (1): 110-5. [ Links ]
13. Yao GX, Xue XB, Jiang ZM, Yang NF, Wilmore DW. Effects of perioperative parenteral glutamine-dipeptide supplementation on plasma endotoxin level, plasma endotoxin inactivation capacity and clinical outcome. Clinical Nutrition 2005; 24 (4):510-5. [ Links ]
14. Yeh CN, Lee HL, Liu YY, Chiang KC, Hwang TL, Jan YY et al. The role of parenteral glutamine supplement for surgical patient perioperatively: result of a single center, prospective and controlled study. Langenbecks Archives of Surgery 2008; 393 (6): 849-55. [ Links ]
15. Fan Y, Yu J, Kang W, Zhang Q. Effects of glutamine supplementation on patients undergoing abdominal surgery. Chin Med Sci J 2009; 24 (1): 55-9. [ Links ]
16. Asprer J, Llido L, Sinamban R, Schlotzer E, Kulkarni H. Effect on immune indices of preoperative intravenous glutamine dipeptide supplementation in malnourished abdominal surgery patients in the preoperative and postoperative periods. Nutrition 2009; 25 (9): 920-5. [ Links ]
17. Gianotti L, Braga M, Biffi R, Bozzetti F, Mariani L. Perioperative intravenous glutamine supplemetation in major abdominal surgery for cancer: a randomized multicenter trial. Ann Surg 2009; 250 (5): 684-90. [ Links ]
18. Dechelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coeffier M, Hecketsweiler B et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: The French controlled, randomized, double-blind, multicenter study. Critical Care Medicine 2006; 34 (3): 598-604. [ Links ]
19. Mercadal Orfila G, Llop Talaverón J, Gracia García B, Martorell Puigserver C, Badía Tahull M, Tubau Molas M et al. Glutamine use for parenteral nutrition in the critically ill patient: effects on morbimortality. Nutr Hosp 22 (1): 61-7. [ Links ]
20. Bakalar B, Duska F, Pachl J, Fric M, Otahal M, Pazout J et al. Parenterally administered dipeptide alanyl-glutamine prevents worsening of insulin sensitivity in multiple-trauma patients. Critical Care Medicine 2006; 34 (2): 381-6. [ Links ]
21. Van den Berghe G, Mesotten D, Vanhorebeek I. Intensive insulin therapy in the intensive care unit. CMAJ 2009; 180 (8): 799-800. [ Links ]
22. Bonet A GT, Piñeiro L, Miñambres E, Acosta J, Robles A, Irles JA, Palacios V, Martínez P. La nutrición parenteral total con dipéptido de glutamina disminuye la infección nosocomial de los pacientes de cuidados intensivos: un estudio prospectivo, aleatorio, de doble ciego y multicéntrico. Nutr Hosp 2008; 23: 2. [ Links ]
23. Wooley JA BI, Good KL. Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract 2005; 20 (2): 176-91. [ Links ]
24. Mertes N, Schulzki C, Goeters C, Winde G, Benzing S, Kuhn KS et al. Cost containment through L-alanyl-L-glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective randomized double-blind controlled study. Clinical Nutrition 2000; 19 (6): 395-401. [ Links ]
25. Alpers DH. Glutamine: Do the data support the cause for glutamine supplementation in humans? Gastroenterology 2006; 130 (2): S106-S16. [ Links ]
26. Griffiths RD, Jones C, Palmer TEA. Six-month outcome of criti cally ill patients given glutamine-supplemented parenteral nutrition. Nutrition 1997; 13 (4): 295-302. [ Links ]
27. Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: Influence of glutamine-supplemented parenteral nutrition on acquired infection. Nutrition 2002; 18 (7-8): 546-52. [ Links ]
28. Jacobi CA OJ, Zuckermann H,. The influence of alanyl-glutamine on immunologic functions and morbidity in post-operative total parenteral nutrition: preliminary results of a prospective randomized trial. Zentralbl Chir 1999; 124: 199-205. [ Links ]
29. Goeters C, Wenn A, Mertes N, Wempe C, Van Aken H, Stehle P et al. Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Critical Care Medicine 2002; 30 (9): 2032-7. [ Links ]
30. PF. Metabolic and therapeutic aspects of amino acids in clinical nutrition. Raton B, editor. L.A. 2004. [ Links ]
![]() Correspondence:
Correspondence:
Gabriel Mercadal Orfila.
Servicio de Farmacia.
Mateu Orfila Hospital.
Avinguda Malbuger, 1.
07701 Maó, Menorca (Spain).
E-mail: gabriel.mercadal@hgmo.es
Recibido: 22-III-2011.
Aceptado: 4-IV-2011.