My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.26 suppl.2 Madrid Nov. 2011
Guidelines for specialized nutritional and metabolic support in the critically-ill patient. Update. Consensus SEMICYUC-SENPE: Acute renal failure
Recomendaciones para el soporte nutricional y metabólico especializado del paciente crítico. Actualización. Consenso SEMICYUC-SENPE: Insuficiencia renal aguda
J. López Martíneza, J. A. Sánchez-Izquierdo Rierab and F. J. Jiménez Jiménezc
aHospital Universitario Severo Ochoa. Madrid. Spain.
bHospital Universitario 12 de Octubre. Madrid. Spain.
cHospital Universitario Virgen del Rocío. Sevilla. Spain.
ABSTRACT
Nutritional support in acute renal failure must take into account the patient's catabolism and the treatment of the renal failure. Hypermetabolic failure is common in these patients, requiring continuous renal replacement therapy or daily hemodialysis.
In patients with normal catabolism (urea nitrogen below 10 g/day) and preserved diuresis, conservative treatment can be attempted. In these patients, relatively hypoproteic nutritional support is essential, using proteins with high biological value and limiting fluid and electrolyte intake according to the patient's individual requirements. Micronutrient intake should be adjusted, the only buffering agent used being bicarbonate.
Limitations on fluid, electrolyte and nitrogen intake no longer apply when extrarenal clearance techniques are used but intake of these substances should be modified according to the type of clearance. Depending on their hemofiltration flow, continuous renal replacement systems require high daily nitrogen intake, which can sometimes reach 2.5 g protein/kg. The amount of volume replacement can induce energy overload and therefore the use of glucose-free replacement fluids and glucosefree dialysis or a glucose concentration of 1 g/L, with bicarbonate as a buffer, is recommended.
Monitoring of electrolyte levels (especially those of phosphorus, potassium and magnesium) and of micronutrients is essential and administration of these substances should be individually-tailored.
Key words: Acute renal failure. Nutritional requirements. Extrarenal clearance.
RESUMEN
El soporte nutricional en la insuficiencia renal aguda está condicionado por el catabolismo del paciente y por el tratamiento del fallo renal. En el paciente crítico es frecuente el fracaso hipermetabólico que obliga a técnicas continuas de reemplazo renal o a hemodiálisis diarias. En los enfermos con catabolismo normal (aparición de nitrógeno ureico inferior a 10 g/día) y diuresis conservada se puede intentar un tratamiento conservador. En estos casos es preciso realizar un soporte nutricional relativamente hipoprotéico, con proteínas de alto valor biológico y limitaciones hidroelectrolíticas individualizadas. Es necesario un ajuste del aporte de micronutrientes, siendo el bicarbonato el único buffer utilizado.
Cuando se utilizan técnicas de depuración extrarrenal desaparecen las limitaciones a los aportes hidroelectrolíticos y nitrogenados, pero éstos deben ser modificados en función del tipo de depuración. Los sistemas continuos de reemplazo renal, en función de su flujo de hemofiltración, precisan altos aporte nitrogenados diarios que en ocasiones pueden alcanzar los 2,5 g de proteínas/kg. La cuantía de la reposición de volumen puede inducir sobrecargas energéticas, siendo recomendable utilizar líquidos de reposición y diálisis sin glucosa o con una concentración de glucosa de 1 g/l, con bicarbonato como buffer.
Es preciso monitorizar los valores de electrolitos (sobre todo de fósforo, potasio y magnesio) y de micronutrientes, y realizar aportes individualizados.
Palabras clave: Insuficiencia renal aguda. Necesidades nutricionales. Depuración extrarrenal.
Introduction
Acute renal failure has become increasingly common in critically-ill patients, related to factors such as hypotension or shock, aging of the population, use of nephrotoxic drugs (antibiotics, antifungals, combination of antihypertensives and antiinflammatories), multiple examinations with radiocontrasts and as organic failure in multiple organ system failure1 (IIb).
Nutritional support in acute renal failure is aimed at preserving lean mass and energy reserve, preventing malnutrition, re-establishing an appropriate immune status, and reducing mortality, attenuating inflammatory response and oxidative stress, and improving endothelial function2. The lack of large adequately designed studies has precluded a high level of evidence on the recommendations. The heterogeneity of the patient group with renal failure requires a standardization that is to be established with the RIFLE classification (Risk, Injury, Failure, Loss, and End-stage kidney)3.
Some years ago, water-electrolyte disorders and intolerance to substrate supply involved a frequently insurmountable stumbling block. Currently, treatment stratification by protein catabolism and diuresis, and the application of continuous and discontinuous renal replacement therapy techniques, based on the characteristics of each patient, allow for an adequate nutritional support.
Nutritional support in acute renal failure is related to the catabolism of the underlying disease, the type of treatment provided, the renal replacement technique used, and the presence of previous malnutrition, and is poorly modified by renal failure itself. Catabolism and treatment are essential for the composition of artificial nutrition. In general, patients with normal catabolism receive conventional treatment, stable patients with a moderately increased catabolism are treated with intermittent hemodialysis, and those with a hypercatabolic status are treated with continuous renal replacement techniques.
What are the protein needs and characteristics of their supply?
In these patients protein catabolism should be calculated by the "appearance of urea nitrogen" (AUN) (Table I), which allows for measuring the amount of urea nitrogen (in urine, in the dialyzate and retained due to lack of clearance) generated in catabolic processes4. In general, patients with AUN < 5 g/day will receive 0.6-0.8 g of protein/kg/day, and will be treated conservatively if they keep diuresis. Patients with AUN between 5 and 10 g/day require protein supplies of 0.8-1.2 g/kg/day. Based on diuresis and on electrolyte disorders they will receive conservative treatment or extrarenal clearance. When the AUN is > 10 g/day, these patients must receive 1.2-1.5 (and sometimes up to 2.5) of proteins/kg/day. They require hemodialysis or continuous renal replacement techniques based on their hemodynamic stability5,6 (IV).
Conservative treatment
Supply must include essential and non-essential amino acids, recommending hypoproteic (up to 1.0 g protein/kg/day) diets (oral or enteral nutrition) with at least 20% of proteins with a high biological value. Exclusive supplies of essential amino acids and histidine are not recommended7 (IIb).
Hemodialysis and peritoneal dialysis
These techniques allow for a protein supply without restrictions, but cause losses leading to increase the requirements. While protein catabolism degree is highly variable from one patient to another, they are usually patients with moderate hypercatabolism. Intermittent hemodialysis causes a loss of amino acids and peptides of 8-12 g and 1-3 g, respectively, in each session. In addition, depending on the biocompatibility of filters, an increase in inflammatory response may occur. Peritoneal dialysis causes daily protein losses of 13-14 g of proteins, that may increase to 18-20 g if peritoneal irritation occurs and exceed 100 g in severe peritonitis. Supplies of 1.2-1.4 g of proteins/kg/day are recommended in hemodialysis8 (IV) and 1.2-1.5 g/kg/day in peritoneal dialysis. Diets and mixtures of standard amino acids are usually adequate in most patients9 (IV).
Continuous renal replacement techniques
Continuous renal replacement techniques are applied to hypercatabolic renal failure requir ing supplies of 1.3-1.5 g of proteins/kg/day, to which losses secondary to the technique used should be added. Studies by Davies10 (IIb) on continuous arteriovenous hemofiltration and Frankenfield11 (IIb) on venovenous hemofiltration verified daily losses of 10-15 g amino acids in the ultrafiltrate, with a negative glutamine balance (as this accounts for 16% of amino acids of the ultrafiltrate). In septic patients high-flow (more than 35 ml/kg/h)12 (IV) and very high flow13 hemofiltration techniques (III) are used, with higher losses. While Frankenfield, Klein and Druml14 consider it is adequate to provide 1.5 g of proteins/kg/day, Bellomo15 (III) and Scheinkestel16 (Ib), 17 (IIa) recommend supplies of 2.2-2.5 g/kg/day, particularly in continuous high-flow hemofiltration. The need for supplementing them with glutamine is discussed.
What are the energy requirements in acute renal failure?
Acute renal failure does not increase per se the energy requirements, and there may even be a "renal hypocatabolism", particularly in extrarenal clearance, for the hypothermia induced by these techniques. The requirements are established by indirect calorimetry or are calculated multiplying resting energy expenditure (REE) by 1.1-1.2. In the practice they correspond to 25-35 total kcal/kg/day18 (IIb).
Conservative treatment
The diets or mixtures used will be rich in carbohydrates to limit hyperkalemia, hyperphosphatemia, and hypomagnesemia, that are common in these patients. Supplies of 25 kcal/kg body weight/day19 (IV) are recommended, with cholesterol-low diets, and a lipid supply of < 1.2 g/kg/day. Occurrence of hypertriglyceridemia limits the amount of calorie intake.
Hemodialysis and peritoneal dialysis
Hemodialysis induces glucose losses, of approximately 25 g per session, while peritoneal dialysis, depending on glucose or polyglucose concentration in the dialysis fluid used, causes a significant glucose and lactate entry, that must be considered when measuring supplies. Patient age is important, and in those over 65 years, 30 kcal/kg/day should not be exceed18 (IIb), 20 (IV).
Continuous renal replacement techniques
The most commonly used are continuous venovenous hemofiltration, requiring a high amount of reple nishment fluid and continuous venovenous hemodia filtration, requiring infusions of replenishment and dialysis fluid. As compared to mandatory daily loses of 25 g of glucose, inappropriate replenishment and dialysis fluids can include major glucose and lactate supplies21 (IIb). Solutions free from glucose or with 1 g of glucose/L are recommended, with bicarbonate as buffer.
Energy supply should be adjusted to the stress level. As they are almost always hypercatabolic clinical states, protein supply must be high, with a low calorie/nitrogen ratio, limiting the energy needs to 25-35 total kcal/kg/day22 (IIb).
What electrolyte and micronutrient supplies do patients with acute renal failure require?
The volume restriction is a limiting factor in acute renal failure on conservative treatment, but renal replacement techniques allow for liberalizing supplies and controlling water balance.
Electrolyte control
Conservative treatment requires close monitoring of sodium supply and controlling hyperkalemia, hypermagnesemia, hyperphosphatemia and metabolic acidosis. Extrarenal clearance techniques can maintain sodium, potassium, and bicarbonate within normal ranges (provided dialysis baths and replenishment fluids with bicarbonate and low lactate content are used). In hypermetabolic renal failure, continuous renal replacement techniques obtain better adjustments than intermittent hemodialysis23 (IIa).
With regards to calcium, hypercalcemia may occur in the intermittent systems and hypocalcemia with continuous techniques, but in the practice they are only clinically relevant when citrate is to be used as system anticoagulant24 (IIb).
The changes in phosphate values are more relevant. In the conservative treatment and intermittent hemodialysis (and in general in all systems using only the diffusion mechanism), hyperphosphatemia is very common. Nutritional support should be low in phosphates. On the contrary, continuous renal replacement techniques based on the convective mechanism cause major phosphate losses. Replenishment fluids are low in phosphorus to prevent their interaction with calcium and bicarbonate. A close monitoring of serum phosphorus levels is essential to detect severe hypophosphatemia and administer the appropriate supplements25 (IIb).
Micronutrient supply
Trace elements are comprised in enzyme systems or in proteins, and their losses with extrarenal clearance systems are mild. Standard supplies are recommended in all patients with renal failure. Selenium values are reduced in critically-ill patients, with and without renal failure26 (Ib). Due to their high antioxidant effect, high supplies are recommended in patients with continuous renal replacement techniques, though they may cause intoxication by selenates. Zinc is low in critically-ill patients, and its deficiency is enhanced with continuous hemofiltration. It must be supplemented, though the standard doses are sufficient27 (IIa). Iron will be supplied in hyposideremia with low ferritin, but not in inflammation and in oxidative stress, with high ferritin28 (IIb).
Water-soluble vitamins should be provided at standard doses in conservative treatment and in intermittent dialysis and double doses in patients with continuous procedures. The fear of causing oxalosis with administration of megadoses of vitamin C, limiting supply to 50 mg/day, explains the low values of this vitamin (very important antioxidant) in critically-ill patients, worsening with continuous hemofiltration. Low thiamine values are common despite supplements26 (Ib).
Fat-soluble vitamins should be administered at standard doses, though in renal failure on conservative treatment or intermittent hemodialysis the dose of vitamin A should be reduced27 (IIa).
Is there a specific nutritional formula for patients with acute renal failure? Do they require specific nutrients?
In non-hypercatabolic renal failure on conservative treatment or intermittent hemodialysis for oligoanuria, standard diets are inadequate due to their low density and excessive contents of sodium, potassium, and phosphates. Low- or normal-protein diets, with high biological value proteins, high energy density and low content in sodium, potassium and phosphates are recommended. With hemodialysis, normal diets may be used, but sometimes phosphorus chelating agents should be administered. A nitrogen supply only with essential amino acids and histidine is currently not indicated29 (Ib).
Hypercatabolic patients, on daily dialysis or continuous renal replacement procedures, may be nourished with a high-protein diet, adjusted to the underlying disease30. Its composition should be based on the essentiality of some amino acids, requiring in some cases to increase the supplies of tyrosine, taurine, histidine, and branched-chain amino acids. In critically-ill patients, the underlying disease would justify using diets with pharmaconutrients in some cases. With hemofiltration, particularly if high or very high flows are used, the appropriateness of supplementing diets (or parenteral mixtures) with glutamine31 (IV), 32 (IIb), should be considered, though the contraindication of administration in non-dialyzed renal failure persists.
What is the recommended supply route in acute renal failure?
Whenever possible nutritional support shall be administered by digestive route. Many patients with low catabolism can tolerate oral diet, alone or with supplements, but critically-ill patients usually require enteral nutrition. If there is any contraindication to it, total parenteral nutrition is to be preferred, with glutamine supplements. As soon as gastrointestinal tract is operative, enteral support will be started, as enteral nutrition is an independent predictor of good prognosis33 (IIb).
Some circumstances may modify this general criterion.
- Highly catabolic patients using continuous highflow renal replacement usually require mixed support, since the major supplies make enteral support insufficient, particularly in the first few days of early nutrition34 (IV).
- Sometimes, the low catabolism of some patients will allow for special parenteral nutritions. One of them is nutritional hemodialysis, using hemodialysis sessions to administer nutrients added to the dialyzate35 (IIb). This leads to reducing the dialyzer flow and is poorly effective in seriously critically-ill patients, but may be of value in patients on continuous hemodialysis and even on slow continuous ultrafiltration (SCUF). Another of them, in patients with non-hypermetabolic acute renal failure and stable hemodynamics, is nutrition by peritoneal dialysis, with dialysis solutions with glucose or polyglucose and amino acids for absorption in the peritoneum. It is usually inadequate in criticallyill patients36 (IIa).
When should nutritional support be started in acute renal failure?
It depends on the catabolism of the patient. With a low catabolism, without prior malnutrition, you may wait until a good oral or enteral tolerability is obtained, after correcting water-electrolyte disorders using fluid therapy. Critically-ill hypercatabolic patients on continuous renal replacement techniques should receive early artificial nutrition, since their underlying catabolism is associated with losses secondary to the clearing technique used. The need to start support very early may advise to start mixed nutrition (enteral and parenteral)37 (IV).
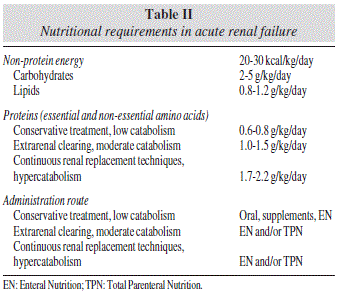
Table II gives a summary of nutritional support in renal failure.
Recommendations
- Protein supply should be adapted to the clinical condition, to the degree of catabolism, and treatment (conservative or extrarenal clearance) performed (B).
- Mixtures of amino acids containing only essential amino acids and histidine should not be used (B).
- Proteins of a high biological value are recommended in non-catabolic patients on conservative treatment (C).
- When extrarenal clearing techniques are used, protein supply should be increased (B). The recommended maximum amount is 2.5 g/kg/day (C).
- With the continuous renal replacement techniques glutamine and taurine supplements are recommended (C).
- In patients on continuous renal replacement techniques, glucose-free replenishment and dialysis solutions or those containing 1 g of glucose/L, with bicarbonate as buffer, are recommended (B).
- Electrolyte (phosphorus, potassium and magnesium) and micronutrient values (zinc, selenium, thiamine, folic acid and vitamin C, A and D) must be monitored, individualizing their supplies (C).
- Standard nutrient supply involves no problems in patients with normal catabolism undergoing clearing procedures (C).
- Although enteral (or oral) nutrition is the method of choice, sometimes the clinical condition of the patient leads to performing parenteral or mixed nutrition (C).
Conflict of interests
The authors declare that they have participated in activities funded by the pharmaceutical industry for marketing of nutritional products (clinical studies, educational programmes and attendance to scientific events). No pharmaceutical industry has participated in the preparation, discussion, writing, and establishing of evidences in any phase of this article.
References
1. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813-8. [ Links ]
2. Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, et al. ESPEN Guidelines on Parenteral Nutrition: adult renal failure. Clin Nutr 2009; 28: 401-14. [ Links ]
3. Valencia E, Marín A, Hardy G. Nutrition therapy for acute renal failure: a new approach based on "risk, injury, failure, loss, and end-stage kidney" classification (RIFLE). Curr Opin Clin Nutr Metab Care 2009; 12: 241-4. [ Links ]
4. Ganesan MV, Annigeri RA, Shankar B, Rao BS, Prakash KC, Seshadri R, et al. The protein equivalent of nitrogen appearance in critically ill acute renal failure patients undergoing continuous renal replacement therapy. J Ren Nutr 2009; 19: 161-6. [ Links ]
5. Jiménez Jiménez FJ, López Martínez JA, Sánchez-Izquierdo Riera J. Nutrición artificial en la insuficiencia renal aguda. Nutr Hosp 2005; 20 (Suppl. 2): 18-21. [ Links ]
6. Bagshaw SM, Cruz DN, Gibney RT, Ronco C. A proposed algorithm for initiation of renal replacement therapy in adult critically ill patients. Crit Care 2009; 13: 317. [ Links ]
7. Druml W, Bürger U, Kleinberger G, Lenz K, Laggner A. Elimination of amino acids in acute renal failure. Nephron 1986; 42: 62-7. [ Links ]
8. Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, Ferrer M et al. An official ATS/ERS/ESICM/SCCM/SRLF Statement: prevention and Management of Acute Renal Failure in the ICU patient: an international consensus conference in intensive care medicine. Am J Respir Crit Care Med 2010; 181: 1128-55. [ Links ]
9. Fouque D, pelletier S, Guebre-Eqziabher F. Have recommended protein and phosphate intake recently changed in maintenance hemodialysis? J Ren Nutr 2011; 21: 35-8. [ Links ]
10. Davies Sp, Reaveley DA, Brown EA, Kox WJ. Amino acid clearances and daily losses in patients with acute renal failure treated by continuous arteriovenous hemodialysis. Crit Care Med 1991; 19: 1510-5. [ Links ]
11. Frankenfield DC, Badellino MM, Reynolds HN, Wiles CE 3rd, Siegel JH, Goodarzi S. Amino acid loss and plasma concentration during continuous hemodiafiltration. JPEN J Parenter Enteral Nutr 1993; 17: 551-61. [ Links ]
12. Luyckx VA, Bonventre JV. Dose of dialysis in acute renal failure. Semin Dial 2004; 17: 30-6. [ Links ]
13. Uchino S, Bellomo R, Goldsmith D, Davenport P, Cole L, Baldwin I, et al. Super high flux hemofiltration: a new technique for cytokine removal. Intensive Care Med 2002; 28: 651- 5. [ Links ]
14. Druml W, Kierdorf HP; Working group for developing the guidelines for parenteral nutrition of the German Association for Nutritional Medicine. Parenteral nutrition in patients with renal failure. Guidelines on Parenteral Nutrition. Chapter 17. Ger Med Sci 2009; [ Links ] 18: 7.
15. Bellomo R, Tan HK, Bhonagiri S, Gopal I, Seacombe J, Daskalakis M et al. High protein intake during continuous hemodiafiltration: impact on amino acids and nitrogen balance. Int J Artif Organs 2002; 25: 261-8. [ Links ]
16. Scheinkestel CD, Kar L, Marshall K, Bailey M, Davies A, Nyulasi I et al. Prospective randomized trial to assess caloric and protein needs of critically Ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition 2003; 19: 909- 16. [ Links ]
17. Scheinkestel CD, Adams F, Mahony L, Bailey M, Davies AR, Nyulasi I, et al. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition 2003; 19: 733-40. [ Links ]
18. Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Picetti E, Parenti E et al. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant 2005; 20: 1976-80. [ Links ]
19. Guimarães SM, Cipullo JP, Lobo SM, Burdmann EA. Nutrition in acute renal failure. Sao paulo Med J 2005; 123: 143-7. [ Links ]
20. Fiaccadori E, Parenti E, Maggiore U. Nutritional support in acute kidney injury. J Nephrol 2008; 21: 645-56. [ Links ]
21. Frankenfield DC, Reynolds HN, Badellino MM, Wiles CE 3rd. Glucose dynamics during continuous hemodiafiltration and total parenteral nutrition. Intensive Care Med 1995; 21: 1016- 22. [ Links ]
22. Macias WL, Alaka KJ, Murphy MH, Miller ME, Clark WR, Mueller BA. Impact of the nutritional regimen on protein catabo lism and nitrogen balance in patients with acute renal failure. JPEN J Parenter Enteral Nutr 1996; 20: 56-62. [ Links ]
23. Uchino S, Bellomo R, Ronco C. Intermittent versus continuous renal replacement therapy in the ICU: impact on electrolyte and acid-base balance. Intensive Care Med 2001; 27: 1037-43. [ Links ]
24. Klein CJ, Moser-Veillon PB, Schweitzer A, Douglass LW, Reynolds HN, Patterson KY et al. Magnesium, calcium, zinc, and nitrogen loss in trauma patients during continuous renal replacement therapy. JPEN J Parenter Enteral Nutr 2002; 26: 77-92. [ Links ]
25. Troyanov S, Geadah D, Ghannoum M, Cardinal J, Leblanc M. Phosphate addition to hemodiafiltration solutions during continuous renal replacement therapy. Intensive Care Med 2004; 30: 1662-5. [ Links ]
26. Berger MM, Shenkin A, Revelly JP, Roberts E, Cayeux MC, Baines M et al. Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am J Clin Nutr 2004; 80: 410-6. [ Links ]
27. Story DA, Ronco C, Bellomo R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med 1999; 27: 220-3. [ Links ]
28. López Martínez J, Temprano Vázquez S, Sánchez Castilla M, Algora Weber A, Jiménez Jiménez J, Del Nogal Sáez F. Metabolismo del hierro en el paciente crítico. Med Intensiva 1995; 19: 285-92. [ Links ]
29. Mirtallo JM, Schneider PJ, Mavko K, Ruberg RL, Fabry PJ. A comparison of essential and general amino acid infusions in the nutritional support of patients with compromised renal function. JPEN J Parenter Enteral Nutr 1982; 6: 109-13. [ Links ]
30. Brow RO, Compher C; American Society for Parenteral and Enteral Nutrition (ASPEN), Board of Directors. ASPEN Clinical guidelines: nutrition support in adult acute and chronic renal failure. JPEN J Parenter Enteral Nutr 2010; 34: 366-77. [ Links ]
31. Fiac cadori E, Cremaschi E. Nutritional assessment and support in acute kidney injury. Curr Opin Crit Care 2009; 15: 474-80. [ Links ]
32. Novák I, Srámek V, Pittrová H, Rusavý P, Lacigová S, Eiselt M et al. Glutamine and other amino acid losses during continuous venovenous hemodiafiltration. Artif Organs 1997; 21: 359-63. [ Links ]
33. Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 2002; 30: 2051-8. [ Links ]
34. Druml W. Nutritional management of acute renal failure. Am J Kidney Dis 2001; 37 (Suppl. 2): S89-94. [ Links ]
35. Korzets A, Azoulay O, Ori Y, Zevin D, Boaz M, Herman M, et al The use of intradialytic parenteral nutrition in acutely ill haemodialysed patients. J Ren Care 2008; 34: 14-8. [ Links ]
36. Van Biesen W, Boer W, De Greve B, Dequidt C, Vijt D, Faict D et al. A randomized clinical trial with a 0.6% amino acid/1.4% glycerol peritoneal dialysis solution. Perit Dial Int 2004; 24: 222-30. [ Links ]
37. Scurlock C, Mechanick JI. Early nutrition support in the intensive care unit: a US perspective. Curr Opin Nutr Metab Care2008; 11: 152-5. [ Links ]
![]() Correspondence:
Correspondence:
J. López Martínez.
Hospital Universitario Severo Ochoa.
Madrid. Spain.
E-mail: lofish@terra.es