My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.27 n.1 Madrid Jan./Feb. 2012
Dietary fibre and cardiovascular health
Fibra dietética y salud cardiovascular
F. J. Sánchez-Muniz
Departamento de Nutrición y Bromatología I (Nutrición). Facultad de Farmacia. Universidad Complutense. Madrid. España.
ABSTRACT
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in developed countries. CVD is an inflammatory disease associated with risk factors that include hypercholesterolemia and hypertension. Furthermore, the evolution of this disease depends on the amount of modified lipoproteins (e.g. oxidized) present in the arterial subendothelium. Diet is considered the cornerstone for CVD treatment, as it can lower not only atherogenic lipoprotein levels and degree of oxidation, but also blood pressure, thrombogenesis and concentrations of some relevant factors (e.g. homocystein).Among different diets, the Mediterranean diet stands out due to their benefits on several health benefits, in particular with regard to CVD. Rich in vegetable foods, this diet contributes both quantitatively and qualitatively to essential fibre compounds (cellulose, hemicellulose, gums, mucilages, pectins, oligosaccharides, lignins, etc.). The present paper analyzes the effects of fibre consumption on a) cholesterol and lipoprotein levels; b) systolic and diastolic blood pressures; and c) antioxidant availability and profile. Some studies and meta-analysis are revised, as the possible mechanisms by which fibre may decrease plasma total cholesterol and LDL-cholesterol and blood pressure and to act as antioxidant, as well. In addition, author´s own publications regarding the effect of fibre matrix (e.g. seaweeds) on arylesterase and the gene expression of some key antioxidant enzymes are reviewed. The paper also includes data concerning the possible interaction between fibre and some hypolipemic drugs, which may make it possible to attain similar hypolipemic effects with lower dosages, with the consequent decrease in possible side effects. The review concludes with a summary of nutritional objectives related to the consumption of carbohydrates and fibre supplements.
Key words: Fibre. Cholesterol. Lipoproteins. Blood pressure. Antioxidants.
RESUMEN
La enfermedad cardiovascular (EC) sigue siendo la causa más frecuente de morbi-mortalidad en los países desarrollados. Es considerada una enfermedad inflamatoria asociada a la presencia de algunos factores de riesgo como hipercolesterolemia e hipertensión. El desarrollo de la EC depende de la cantidad de lipoproteínas modificadas (p.e. oxidadas) presente a nivel del espacio subendotelial arterial. La dieta se considera piedra angular en el tratamiento de la EC, ya que no sólo puede reducir el nivel y la oxidación de las lipoproteínas aterogénicas, la presión arterial sino el impacto de algunos factores relevantes (p.e. trombogénesis, homocisteína). Resalta entre muchas dietas la tipo mediterráneo debido a sus beneficios contrastados en múltiples aspectos de salud. La dieta mediterránea contiene una amplia variedad de vegetales que aportan la mayoría de los componentes (celulosa, hemicelulosa, gomas, mucílagos, pectinas, oligosacáridos, ligninas, etc) que integran el concepto de fibra tanto cuantitativa como cualitativamente. De algunos de ellos hay clara evidencia científica de sus beneficios bajo el punto de vista de la EC. La revisión se ha estructurado en los siguientes puntos que analizan los efectos de la fibra dietética sobre a) el contenido plasmático de colesterol y lipoproteínas; b) la presión arterial sistólica y diastólica y c) la capacidad y perfil antioxidante. Se revisan algunos estudios y meta-análisis, se proponen posibles mecanismos por los que la fibra dietética disminuye los niveles de colesterol total, LDL-colesterol y presión arterial y actúa como antioxidante. Además se revisan algunas publicaciones propias sobre los efectos de una matriz fibrosa (algas) sobre la actividad de la arilesterasa y la expresión génica de enzimas antioxidantes clave. El artículo también revisa la posible interacción de la fibra con fármacos hipolipemiantes. Esta interacción permitiría reducir la dosis del fármaco y los posibles efectos secundarios. La revisión termina con algunos objetivos nutricionales relacionados con el consumo de alimentos ricos en hidratos de carbono y fibra dietética.
Palabras clave: Fibra dietética. Colesterol. Lipoproteínas. Presión arterial. Antioxidantes.
Abbreviations
AE: Arylesterase.
CI: Confidence interval.
CHD: Coronary heart disease.
CVD: Cardiovascular disease.
HDL: High density lipoproteins.
LDL: Low density lipoproteins.
rLDL: LDL receptors.
MI: Miocardial infarction.
RR: Relative risk.
SOD: Superoxide dismutase.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death and disability in developer countries1 and was predicted by the WHO to become the major cause of mortality in developing countries by 2010.2
Atherosclerosis, a process characterized by endothelial dysfunction,3 is associated with several risk factors such as hypertension,4 smoking,5 and hypercholesterolemia.6 At present CVD is considered an inflammatory disease. In its initial phase cholesterol and free radical deposition in macrophages and smooth cells in the arterial vessel take place. This process is related to elevated presence of low-density lipoproteins (LDL),3,7 Lp(a),8 and lipoprotein remnants [e.g. remnants of chylomicrons, intermedium lipoproteins (IDL)], at the subendothelial arterial level.7 This process is also characterized by local inflammation, myocyte proliferation and vascular calcification.3 Most of the times, arterial thrombosis, which is associated with plaque disruption and aggravated by high levels of fibrinogen and coagulation factors and vasoconstriction, represents a terminal event due to vascular stenosis or occlusion.3
Arterial blood pressure is a key factor in the development of CVD. In fact, the WHO recognizes arterial hypertension as the most prevalent CVD in the world.1 Hypercholesterolemia appears to be another critical factor, as CVD is uncommon in societies in which blood cholesterol levels is low.6 Smoking, which induces arrhythmia and/or alterations of the lipoprotein profile and thrombogenic factors, has long been recognized as a major CVD risk factor.5,9
It has recently been suggested that insulin resistance is a key contributor to the metabolic syndrome, a cluster of medical conditions in which overweight/obesity, dislipemia, hyperglycemia, and hypertension also play important roles and highly contributes to VD risk.10,11 Insulin resistance is related to the presence of TNFα, cortisol, glucagon, and other factors that decrease glucosetolerance, induce an atherogenic lipid profile (phenotype B LDL), and increase insulinemia. In turn, among other effects, insulinemia activates the sympathetic nervous system and promotes Na retention and smooth muscle cell proliferation10-12 (fig. 1).
Controlling the modifiable risk factors can help prevent CVD. Both the lipoprotein profile and blood pressure, which clearly affect endothelial function and arterial health, can be improved through dietotherapy.
Table I summarized central dietary that must be considered in the prophylaxis and treatment of CVD.13,14 The adequate intake of dietary fibre through the high consumption of fruits and vegetables characteristic of the Mediterranean diet15 makes possible to achieve all four objectives enumerated in table I (healthy diet, correct body weight, acceptable lipoprotein profile, and normal blood pressure).
Notwithstanding the numerous attempts to clarify the concept of dietary fibre since the 1970´s, no consensus has yet been reached with regard to the definition of this term. One of the most frequently cited definitions is (sic) "Dietary fibre is the edible parts of plants or analogous carbohydrates that are resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. Dietary fibre includes polysaccharides, oligosaccharides, lignin and associated plant substances".16 In addition "analogous carbohydrates" is defined as those carbohydrates-based food ingredients that are non-digestible and non-absorbable, and which are similar to plant dietary fibre for which most of the dietary fibre research has encompassed".16 The numerous compounds of which the different types of fibre are composed, and certain elements associated with fibre (e.g. polyphenols, minerals), make it necessary to accurately speak of dietary fibres in the plural, despite the fact that fibre is considered a biological entity. The distinct composition of the different fibre types explains the plurality of functions ascribes to this food component, including their water retention capacity, absorption properties (bile salt, glucose, and fat absorption binding capacity), tendency to form gels, viscosity, fermentability, and ability to modify gut microbiota composition,17,18,19 these dietary components can affect metabolism and modify certain CVD risk factors, improving the prognosis of CVD and reducing the probability of cardiovascular events (table II).
In addition, dietary fibres present potential functions that were unknown until recently. The science of nutrigenomics deals with the effects of diet, including those of fibres on the genoma.20,21 Diet including fibre, can interact with our microbiota genoma and modify itself gene expression. Moreover, it can be hypothesized that many compounds produced by human microbiota such as enzymes, interleukins and shortchain fatty acids could affect the cellular transcription, translation, and post-translational events of the microbiota and those of neighboring colonocytes (fig. 2).
Ample epidemiological evidence exists from follow- up studies performed in large prospective cohorts that examine the hypothesis that higher intake of dietary fibre is inversely related to the risk of CVD and myocardial infarction (MI).
One study of obliged reference is that of Liu et al.22 that using a semi-quantitative food frequency questionnaire assessed dietary fibre intake among 39,876 female health professionals with no previous history of CVD or cancer. Women were subsequently followed for an average of six years for incidence of nonfatal MI, stroke, percutaneous transluminal coronary angioplasty, coronary artery bypass graft or death due to CVD confirmed by medical records or death certificates. During the follow-up, 570 incident cases of CVD with 177 MIs were documented. A significant inverse association was observed between dietary fibre intake and CVD risk. Comparing the highest quintile of fibre intake (median: 26.3 g/day) with the lowest quintile (median: 12.5 g/day), the relative risks (RR) were 0.65 (95% confidence interval [CI]: 0.51, 0.84) for total CVD and 0.46 (95% CI: 0.30, 0.72) for MI. In this publication22 it also has shown a comparison of the results between dietary fibre intake and CVD risk in 10 known epidemiological studies.23-32 Pooled results suggest that the RR of CVD is reduced 15% for each 10 g dietary fibre consumed. Interestingly, a stronger association was found on CVD protection for cereal fibre than for vegetable or fruit fibres consumptions (fig. 3).
Although CVD is a multifactorial disease in which several factors are involved, this review, nonetheless, deals specifically in the effect of dietary fibres (in this review dietary fibre) on three CVD-key parameters from which adequate scientific literature is available: a) cholesterol metabolism; c) blood pressure; and c) antioxidant properties.
Fibre and cholesterol metabolism
The effects of dietary fibre intake on cholesterol metabolism have been studied extensively, although the molecular mechanisms involved are not completely known. It has been known for years that vegetarians and semi-vegetarians33,34 display lower cholesterol and LDL-cholesterol levels, and lower LDL-cholesterol/ HDL-cholesterol and cholesterol/HDL-cholesterol ratios than omnivores. Time ago, Keys et al.35 established that some types of dietary fibre can decrease plasma total cholesterol in humans, several studies have shown that a high consumption of dietary fibre, particularly fermentable fibre (guar gum, β-glucans, glucomannan, pectin, psyllium), significantly decrease serum total cholesterol and LDL-cholesterol levels.36,3
Martinez de Prado et al.38 studying the effect of partial substitution of saw dust, citrus pectin, cellulose and wheat bran found that only pectin was effective reducing plasma cholesterol in hypercholesterolemic casein rabbits. Neutral steroid excretions were higher in pectin fed rabbits.
Babio et al.39 has recently reviewed some studies that examine the effect of different types and sources of dietary fibre on body weight, glucose metabolism and lipid profile. The authors concluded (sic) that clinical studies consistently show that the intake of viscous dietary fibre decreases the LDL-cholesterol levels.
Figure 4 suggests a mechanism by which certain fibres, including lignin and β-glucans, which apparently act as bile salt sequestrants, may reduce plasma LDL-cholesterol levels. By this mechanism dietary fibres partially block the enterohepatic cycle avoiding the liver bile acids re-use and increasing hepatic expression of limiting enzyme 7α-cholesterol hydroxylase (CYP7A1), which transforms cholesterol into cholic acid. This, in turn, assures production of more bile acids while decreasing the cholesterol available for inclusion in lipoproteins (e.g. VLDL).
The fermentability of fibre compounds ranges between 10%-90%. The least fermentable of these compounds include lignin and cellulose, while pectin, gums, resistant starch, mucilage and some oligosaccharides are highly fermentable. Anaerobic fermentation produces CO2, CH4, H2, and short chain fatty acids (butyric, propionic and acetic acids).17 Production of butyric acid, associated with renewal of microbiota and colonocytes and reduction of intestinal inflammation and risk of neoplasia, increases intestinal health.17 Algal fibres are less fermentable that those of other plant foods,18 probably due to their high P content.
Propionic acid is thought to have hypocholesterolemic properties in rats and pigs,40 but this was not substantiated by further studies.41 Alvarez-Leite et al.42 showed that the reduction in serum cholesterol promoted by guar gum was comparable in germ-free rats and germ-free rats colonized with normal human flora producing short-chain fatty acids. Zhang et al.43 found similar lowering effect of oat bran supplementation in ileostomized hypercholesterolemic individuals, with a virtual suppressed fermentation process, than in subjects with a functional colon. Thus, the mechanism relating fibre fermentability with the decrease in blood cholesterol concentrations in human can not be supported any longer.
Nillson et al.44 determined the plasma concentrations of acetate, propionate, and butyrate in a group of healthy volunteers the morning following intake of 8 different cereal-based evening meals whose indigestible carbohydrate contents varied but which all included 50 g of available starch. Each of the study participants consumed all test meals in random order on separate evenings. Individuals whose evening meals consisted of amylose-rich barley kernels or β-glucan- rich barley kernels presented higher plasma butyrate concentrations than those who consumed white wheat bread. At a standardized breakfast following the evening test meals, the postprandial glucose response (incremental area under the curve, 0- 120 min) was inversely related to plasma butyrate and acetate concentrations. According to the authors, results support the view that cereal products rich in indigestible carbohydrates may improve glucose tolerance through a mechanism involving their colonic fermentation and generation of SCFA, in which butyric acid may play a role. This mechanism may partially explain the protective effect of whole grains against type 2 diabetes and CVD. As mentioned above, insulin resistance is associated with atherogenic phenotype B LDL10,11 (fig. 1).
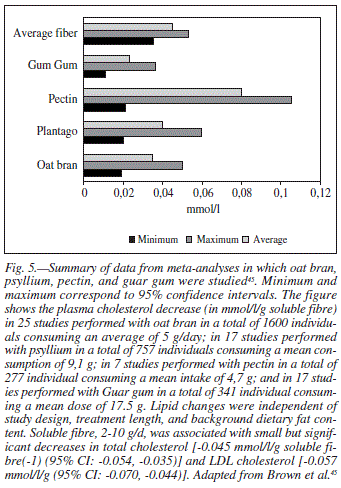
Brown et al.45 performed a meta-analysis on the effect of different dietary fibres on plasma cholesterol. In 25 studies involving 1,660 individuals, an average daily intake of 5 g of oat fibre (oat bran) led to a net decrease in plasma cholesterol of about 0.037 (CI, -0.022, -0.051) mmol/l /g of soluble fibre. These authors also reported that an average consumption of 9.1 g of psyllium per day in 17 studies (757 individuals) decreased plasma cholesterol by about 0.028 (CI, -0.020, -0.037) mmol/ l/g of soluble fibre. Furthermore, in 7 studies involving 277 individuals an average daily intake of 4.7 g of pectin decreased plasma cholesterol by 0.070 (CI, -0022, -0.117) mmol/l/g soluble fibre, while in 17 trials with 342 participants an average daily Guar gum intake of 17.5 g lowered plasma cholesterol levels by about 0.026 (CI, -0.015, -0.035) mmol/l/g of soluble fibre (fig. 5). These authors45 indicated that the effects on plasma lipids of soluble fibre from oat, psyllium, or pectin were not significantly different and triglycerides and HDL cholesterol were not significantly influenced by soluble fibre. They were unable to compare effects of guar because of the limited number of studies using 2-10 g/d. Brown et al.45 concluded that the effect is small within the practical range of intake.
In another meta-analysis of 8 controlled trials, Anderson et al.36 reported that fibre from psyllium (Plantago ovata) lowered serum total cholesterol and LDL cholesterol in average by about 4% and 6%, respectively, compared with the results of a dietary placebo.
More recently, AbuMweis et al.46 have precisely quantified the effect of barley β-glucan on blood lipid concentrations in humans and examined the factors that could affect its efficacy. Eleven eligible randomized clinical trials published from 1989 to 2008 were selected from nine databases. Diets with barley and β-glucan isolated from barley lowered total and lowdensity lipoprotein (LDL) cholesterol concentrations by 0.30 mmol/L and 0.27 mmol/L on the average, respectively, compared with control diets. The pattern of the cholesterol-lowering action of barley in this analysis could not be viewed as a dose-dependent response. There were no significant subgroup differences by type of intervention and food matrix. These authors concluded that a dietary approach to reduce LDL cholesterol concentrations should take into account a higher intake of barley products.
Table III summarizes some studies relating dietary fibre consumption and plasma lipids in ample number of individuals. Lairon et al. in France found in crosssectional studies that the highest dietary intake was associated with lower plasma triglyceride47 and with lower apolipoprotein B, total cholesterol and triglyceride levels.48 Van Dam et al.49 in The Netherlands found that a dietary pattern rich in vegetables and salad was associated with higher HDL cholesterol levels. More recently whole grain consumption has been negatively associated with plasma total cholesterol and LDL cholesterol. Berg et al.50 in Switzerland found that a high dietary fibre diet was associated with low triglyceride and high HDL cholesterol levels. Newby et al.51 in USA compared whole grain, whole grains, refined grains, and cereal fibre. Whole-grain and cereal fibre intakes were associated with lower body mass index and total cholesterol, LDL cholesterol.
For years, our research group has studied the hypocholesterolemic effect of algae,18,52 whose dry matter residue is very rich in fibre.18,19 Ren et al.53 reported that some seaweed polysaccharides exert hypolipemic effects in rats fed a diet rich in sodium and cholesterol. Sodium alginate, funoran, porphyran, and carrageenan interacted with dietary cholesterol to facilitate its excretion, while dietary agar was almost inactive.54 Bocanegra et al.54 found that a Nori supplement displayed modest hypocholesterolemic effects in a cholesterol-enriched diet, while Konbu was unable to even partially block the effect of the dietary hypercholesterolemic agent. Different effects on blood cholesterol levels were observed more recently after inclusion of Nori, Wakame, and Sea Spaghetti in restructured meats.55,56 These data can be associated with CYP7A1 induction.56-58
Effects of dietary fibres on blood pressure
Data concerning the effects of dietary fibres on blood pressure are scarcer than those available on their effects on cholesterol levels, and little is known regarding the mechanisms involved at the present time. It has been suggested that diet (and in turn fibre) by modifying concentrations of LDL and HDL affects arterial endothelia and smooth muscle modifying the arterial contraction tone.59 Nonetheless, it can be speculated that fibre could influence cardiac input/output and total peripheral resistance by affecting the sympathetic and parasympathetic nervous systems or by modifying concentrations of several local or systemic regulators (e.g. K+, CO2, H+, prostaglandins, thromboxanes, NO).60 Considering the renal role in blood pressure control, dietary fibres could affect the angiotensin-converting enzyme (ACE) activity. This enzyme, in turn, contributes to the formation of angiotensin II, a powerful vasoconstrictor that induces formation of the hormones noradrenaline, aldosterone, and vasopressin (also known as antidiuretic hormone, ADH).60 ACE is also involved in converting bradykinin (a vasodilator, natriuretic, and diuretic agent) into inactive degradation products. Dietary fibres are usually associated with antioxidant compounds (e.g. polyphenols) or mineral (major and trace) that may influence the production of regulators of vascular tone including thromboxanes, prostacyclins, serotonin and NO, among others. Moreover, the hypotensive effects of dietary fibres may be associated with the retention of minerals such as K and Mg in their matrix,18 as some minerals have been related to blood pressure.61
Our group also has reviewed the hypotensive properties of seaweeds.19 The effect of algal polysaccharides on blood pressure in rats fed a diet containing 1.5% NaCl, 0.5% bile salts, and 1.5% cholesterol differed according to each polysaccharide. Those that clearly affect blood pressure include fucoidans, alginates and funorane, while glucoronoxyloramnan and porphyran displayed no significant effects at all.
For the present review, the authors selected two meta-analyses, each of which considered several trials dealing with the effect of different types and amounts of fibre on blood pressure. Summary data are presented in table IV. Streppel et al.62 performed a meta-analysis of random placebo-controlled studies to estimate the effect of fibre supplementation on systolic and diastolic blood pressures. Original articles published during 37 years were included in this meta-analysis63-84 (table IV). Data were abstracted on fibre dose, fibre type, blood pressure changes, study design features, and study population characteristics. A random-effects model was used for meta-analysis. The results of this meta-analysis indicate that an average dose of 11.5 g/d fibre supplementation lowered systolic blood pressure by 1.13 mmHg (95% confidence interval: -2.49 to 0.23) and diastolic blood pressure by 1.26 mmHg (CI -2.04 to -0.48). Reductions in blood pressure tended to be greater in older (> 40 years) and hypertensive populations than in younger and normotensive ones. This metaanalysis concluded with the following recommendation (sic) "Increasing the intake of fibre in Western populations, where intake is far below recommended levels, may contribute to the prevention of hypertension".
Whelton et al.85 conducted another similar meta-analysis of 25 randomized controlled trials to assess the effect of dietary fibre intake on blood pressure65-68,70,73-79,81-83,86-93 (table IV). Using a standardized protocol, information was abstracted on study design, sample size, participant characteristics, duration of follow-up and change in mean blood pressure. The data from each study were pooled using a random effects model to provide an overall estimate of dietary fibre intake on blood pressure. On the average dietary fibre intake was associated with a significant 1.65 mmHg reduction in diastolic blood pressure [95% confidence interval (CI) -2.70 to -0.61] and a non-significant 1.15 mmHg reduction (95% CI, - 2.68 to 0.39) in systolic blood pressure. Significant decreases in both systolic and diastolic blood pressures were observed in patients with hypertension (systolic blood pressure -5.95 mmHg; diastolic blood pressure - 4.20 mmHg) and in trials with a duration of intervention ≥ 8 weeks (systolic blood pressure -3.12 mmHg; diastolic systolic blood pressure -2.57 mmHg) (fig. 6). Whelton et al.63 concluded that increased intake of dietary fibre may reduce blood pressure in patients with hypertension and suggested that normotensive individuals who increase their fibre consumption may achieve a small, non-conclusive reduction in blood pressure. An intervention period of at least 8 weeks may be necessary to achieve the maximum reduction in blood pressure.
Maki et al.94 analyzed the effects of consuming foods containing oat β-glucan on blood pressure in hypertensive men and women. The authors followed a randomized, double-blind, controlled clinical trial design. Ninety-seven men and women with resting systolic blood pressure values of 130-179 mmHg and/or diastolic blood pressure levels of 85-109 mm Hg were randomly assigned to consume foods containing oat β-glucan or control foods for 12 weeks. Blood pressure responses were not significantly different between groups. However, individuals with a body mass index > 31.5 kg/m2 in the β-glucan group displayed decreases in systolic (8.3 mmHg) and diastolic (3.9 mmHg) blood pressures than their counterparts in the control group.
Effects on oxidative stress
As previously commented, CVD is an inflammatory condition in which oxidative modifications of lipoproteins play a central role. Dietary fibres could exert a protective effect by quenching or deleting free radicals, interchanging ions, counteracting the negative action of free radicals with antioxidant compounds associated with their polysaccharide matrix.95
Anderson et al.96 systematically reviewed literature from the past 20 years evaluating an association between dietary fibre and coronary heart disase (CHD). Foods that are rich in dietary fibre, including fruits, vegetables, legumes and whole grain cereals, also tend to be a rich source of vitamins, minerals, phytochemicals, antioxidants and other micronutrients. Population-based cohort studies97,98 suggest that small increases in vitamin E intake are associated with significant reductions in CVD. According to Anderson et al.96 (sic) the vitamin and antioxidants provided by whole grain foods may contribute to their cardioprotective effect. Maki et al.94 studied the effect of oat β-glucan on four biomarkers of oxidative stress. No significant effects were found on those biomarkers.
Most effect of fibre can be ascribed to associate compounds such as polyphenols. Thus, Lecumberri et al.95 analyzed the associate polyphenolic content of cocoa fibre and its effect on the antioxidant capacity of rats serum. Cocoa fibre appeared as an excellent source of dietary fibre (about 60% of the dry matter) rich in polyphenols (1.5%). After intragastric administration of the polyphenol-rich extract a fast and measurable amount of polyphenols was detected in blood. The absorption of polyphenols confers a significant, although transitory, increase of the serum antioxidant capacity 10-45 minutes post-gavage. Gómez-Juaristi et al.99 in their review described that the consumption of cocoa/chocolate (rich in polyphenols) increases plasma antioxidant capacity.
Our group has studied the effect of alga consumption on the arylesterase activity of growing rats.52 Arylesterase (AE) activity is one of the major activities of the paraoxonase enzyme (PON-1). This enzyme is associated with HDL and protects LDL from peroxidation. 100,101 Figure 7 shows the effect of a 7% dietary supplement of Nori or Konbu. These algae increased the activity of AE in young rats fed a diet with no supplementary cholesterol. AE activity also increased when the enzyme was standardized with relation to the HDL mass.52 Our research group has recently combined alga with restructured pork in an effort to obtain healthier meat products.55,56
Fibre-enriched foods. Functional foods
The primary function of the diet is to provide the nutrients needed to meet individual´s daily metabolic requirements. Food consumption, furthermore, provides pleasurable feelings of satisfaction and wellbeing through the senses (e.g. taste). In addition, diet can positively affect one or various physiological functions and reduce disease risk. The Scientific Concepts of Functional Foods in Europe: Consensus Document, generated by the European Commission on Functional Food Science in Europe (FUFOSE), suggested (sic) that claims of "enhanced function" and "reduced risk of disease" may only be made when they are supported by scientific data including appropriate, validated markers of exposure, target function or an intermediate endpoint.102
Potential functional foods have been obtained by including different types of fibre to various food matrices (e.g. juices, yogurt, meat, fish). As meat consumption has been epidemiologically related to major degenerative disorders, such as CHD,103 our group has worked to obtain functional meat products.104-108 Alternatives to conventional meat products include low-fat, low-sodium restructured meat with additives such as alga.109
As commented, seaweeds and/or their water-soluble fraction or isolated alga polysaccharides may present antioxidant18,19,56 and hypolipemic properties.18,52,55 Our research group has studied the effects of different dietary seaweed supplements on liver glutathione status in nomal and hypercholesterolemic growing rats. Values of total and reduced reduced glutathione, glutathione reductase activity, plasma cholesterol, and total antioxidant capacity differed between rats consuming Nori and those given Wakame.56 Our group has recently reported that cholesterol-enriched diets with restructured pork containing Wakame affected antioxidant enzyme gene expressions and activities in growing Wistar rats, while those that contained Nori affected plasma cholesterol levels.56
Figure 8 shows that the inclusion of Himantalia elongate (Sea Spaghetti) in this meat increased expression of one of the main antioxidant enzymes, Cu,Znsuperoxide dismutase (Cu,Zn-SOD).110 Intake of restructured pork containing Sea Spaghetti significantly increased SOD expression, while that of the cholesterolenriched meat caused its expression to decline. However, in rats given the restructured pork diet enriched in cholesterol and Sea Spaghetti, SOD expression was similar to that of rats fed the control diet.110
Fibre and hypocholesterolemic drugs
Hypolipemic drugs are extensively used to lower and maintain acceptable blood lipid levels. Statins are a class of commonly used, effective hypolipemic drugs.111-113 However, interaction between certain hypocholesterolemic drugs (e.g. fibrates) and statins may increase the efficacy of the latter, but also the incidence of possible such side effects as liver failure and rhabdomyolysis.114 Our group has recently reviewed the major interactions between statins and some dietary components.113-115 Results of this review indicate that synergism/negative interaction between statins and some major dietary components increases/decreases the hypolipemic effect or the half-life of the drugs. The synergism dietwith statins that increases the efficacy of these drugs may make it possible to reduce the dosage, thus avoiding potential adverse effects. Moreyra et al.116 have reported that simultaneous intake of psyllium and 10 mg of simvastatin produces effects that are similar to those observed with 20 mg of the same drug taken alone on total cholesterol, LDL, Apolipoprotein A1, and HDL cholesterol levels.
Nutritional objectives and conclusions
Published data indicate that the Mediterranean diet protects against numerous disorders.15 Basing their recommendations on the Mediterranean diet pyramid, several nutritional authorities117-119 have made the following guidelines:
Diet should be varied and include 4-6 servings of cereals per day, 5 servings of vegetables/fruit per day, and a minimum of 2 servings of legumes per week. Carbohydrates should contribute over 50% of total energy intake, except in individuals with hypertriglyceridemia, for whom they should contribute about 45%. Consumption of carbohydrates with a low glycemic index in the framework of dietary fibre ensures a diet with a low glycemic load. The energy contribution of simple carbohydrates should be below 10%. Consumption of fructose, as an isolated sugar should be avoided. Total fibre intake should be between 30-40 g per day (10-13 g/1,000 kcal). Thus, a diet of 2,000 kcal may contain about 25 g of fibre, while that of 2,500 kcal about 30 g. According to the Dietary Reference Intakes of USA,118 it has been proposed 38 g/day for males, and 25 g/day for females.
According to the last World Health Organization workshop on carbohydrates and human nutrition, intact fruits, vegetables, whole grains, nuts and pulses are the best dietary fibre sources, because are rich in cardioprotective components.120 Thus, a Mediterranean diet should be recommended to improve the lipoprotein profile and to reduce CVD risk.121,122
Further remarks
Due to the positive effects of dietary fibres on the major CVD risk factors known to date, new studies have to be designed to further our understanding of the benefits of dietary fibres in western, Mediterranean, or hypocaloric diets on emerging CVD risk factors, such as Lp(a), postprandial lipemia, thrombogenesis, blood coagulation factors, inflammation and antioxidant markers. Moreover, further research is needed on the possible plural interactions between dietary fibre, other dietary compounds and colon microbiota, as fibre compounds are known to interact with dietary macro and micronutrients. In addition, as there exists hypo and hyporresponders to diet and drug treatment,21,123,124 more information regarding the fibregene interaction is needed in order to personalize and individualize dietotherapy and pharmacotherapy.
Acknowledgements
The author acknowledges the invitation to participate in the course entitled "Consumo de fibra dietética y salud" behold the 28 and 29 of March 2011 at the Facultad de Farmacia de la Universidad Complutense de Madrid (Spain) and the information given by the Kátedra Kellogg´s as "Fibra dietética y salud cardiovascular. Alimentando el conocimiento". Thanks are also due to the Projects ALI, AGL-2008 04892-C03- 02, and Consolider-Ingenio 2010 # CSD2007-00016.
References
1. Comunidad Europea. Salud Pública. Ficha informativa sobre enfermedades crónicas: enfermedades cardiovasculares. http://www.ec.europa.eu/health/phinformation/dissemination/diseases/#information [ Links ]
2. American Heart Association. Cardiovascular disease statistics. http://www.americanheart.org/presenter.jhtml [ Links ]
3. Ross R. Atherosclerosis an inflammatory disease. N Eng J Med199; 340: 115-126. [ Links ]
4. Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001; 358: 1305-1315. [ Links ]
5. Adnot S. Tobacco: an atherogenic, thrombogenic or spasmogenic factor? Arch Mal Coeur Vaiss 1998; 5: 33-38. [ Links ]
6. Stamler J, Greenland P, Van Horn L, Grundy SM. Dietary cholesterol, serum cholesterol, and risks of cardiovascular and noncardiovascular diseases. Am J Clin Nutr 1998; 67: 488-492. [ Links ]
7. Nakada Y, Kurasawa H, Tohyama J, Inoue Y, Ikewaki K. Increased remnant lipoproteins in patients with coronary artery disease-evaluation utilizing a newly developed remnant assay, remnant lipoproteins cholesterol homogeneous assay (R3em- C). J Atheroscler Thromb 2007; 14: 56-64. [ Links ]
8. Koschinsky ML. Lipoprotein (a) and atherosclerosis: new perspectives on the mechanism of action of an enigmatic lipoprotein. Curr Atheroscler Rep 2005; 7: 389-395. [ Links ]
9. Cuesta C, Sánchez-Muniz FJ, García-La Cuesta A, Garrido R, Castro A, San-Felix B, Domingo A. Effects of age and cigarette smoking on serum concentrations of lipids and apolipoproteins in a male military population. Atherosclerosis 1989; 80: 33-39. [ Links ]
10. Ye J. Obesity, inflammation and the metabolic syndrome In Obesity. Serrano Ríos M, Ordovás JM, Gutiérrez Fuentes JA. Elsevier and Fundacion Lilly, Amsterdam, 2010; pp. 169-188. [ Links ]
11. Badimón L, Hernández Vera R, Vilahur G. Fat tissue, thrombosis and atherosclerosis. In Obesity. Serrano Ríos M, Ordovás JM, Gutiérrez Fuentes JA. Elsevier and Fundacion Lilly, Amsterdam, 2010; pp. 233-244. [ Links ]
12. Rubiés-Prat J. Manejo de la dislipemia en el paciente diabético. En: Enfermedades cardiovasculares. Nutrición, genética y epidemiología. Oya M de, Garcés C. (Eds). Fundación Jiménez Díaz. Universidad Autónoma de Madrid. Doyma SL., Madrid: 2000; pp. 95-106. [ Links ]
13. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Kris Etherton P, Goldberg IJ, Kotchen TA, Lichenstein AH, Mitch WE, Mullis R, Robinson K, Wylie Rosset J, St Jeon S, Suttie J, Tribble DL, Bazarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000; 102: 2284-2299. [ Links ]
14. Sánchez-Muniz FJ, Nus M. Importancia de la interacción dietagenética en la prevención cardiovascular. En: Genética, nutrición y enfermedad. Vaquero P (coordinadora). Instituto Tomás Pascual Sanz y Consejo Superior de Investigaciones Científicas. Madrid, 2008; pp. 125-144. [ Links ]
15. Sánchez-Muniz FJ. Aceite de oliva, clave de vida en la Cuenca Mediterránea. An R Ac Nac Farm 2007; 73: 653-692. [ Links ]
16. Prosky L. What is dietary fibre? A new look at the definition. En: Advanced dietary fibre technology. Cleary BV, Prosky, L. Blackwell ScienceOxford, UK. 2001; pp. 63-88. [ Links ]
17. Cleary BV, Prosky, L. (eds). Advanced dietary fibre technology. Blackwell ScienceOxford, UK. 2001; pp. 1-534. [ Links ]
18. Bocanegra A, Bastida S, Benedí J, Ródenas S, Sánchez-Muniz FJ. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J Med Food 2009; 12: 236-258. [ Links ]
19. Jiménez-Escrig A, Sánchez-Muniz F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr Res 2000; 20: 585-598. [ Links ]
20. Ordovás JM Corella D. La revolución del genoma humano. ¿Qué significa genómica, epigenética, nutrigenética, nutrigenómica, metabolómica? En: Genética, nutrición y enfermedad. Vaquero P (coordinadora). Instituto Tomás Pascual Sanz y Consejo Superior de Investigaciones Científicas. Madrid, 2008; pp 19-32. [ Links ]
21. Sánchez-Muniz FJ, Bastida S. Nutrigenómica y nutrigenética. Desde la expresión génica a las dietas a medida. Nutrición en la Mejora del Bienestar y Capacidad Funcional. En: Magíster Universitario. Nutrición y Dietética para la Promoción de la Salud (on-line). Unidad 5. Módulo 7. Departamento de Nutrición y Bromatología I (Nutrición). Facultad de Farmacia. Universidad Complutense de Madrid (Título Propio). COINSA, Madrid, 2010. [ Links ]
22. Liu S, Buring JE, Sesso HD, Rimm EB, Willett WC, Manson JE. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J Am Coll Cardiol 2002; 39: 49-56. [ Links ]
23. Morris JN, Marr JW, Clayton DG. Diet and heart: a poscript. Br Med J 1977; 2: 1307-1314. [ Links ]
24. Kromhout D, Bosschier EB, de Lezenne Coulander C. Dietary fiber and 10-years mortality form coronary heart disease, cancer and all causes: the Zutphen Study. Lancet 1982; 2: 518-522. [ Links ]
25. Kushi LH, Lew RA, Stare FJ et al. Diet and 20-year mortality from coronary heart disease: the Ireland-Boston Diet-Heart study. N Engl J Med 1985; 312: 811-818. [ Links ]
26. Khaw KT, Barrett-Connor E. Dietary fiber and reduced ischemic heart disease mortality rates in men and women: a 12-year prospective study. Am J Epidemiol 1987; 126: 1093-1102. [ Links ]
27. Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease: The Adventist Health Study. Arch Intern Med 1992; 152: 1416-1424. [ Links ]
28. Humble CG, Malarcher AM, Tyroler HA. Dietary fiber and coronary heart disease in middle-aged hypercholesterolemic men. Am J Prev Med 1993; 9: 197-202. [ Links ]
29. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996; 275: 447-451. [ Links ]
30. Pietinen P, Rimm EB, Korhonen P et al. Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men: the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Circulation 1996; 94: 2720-2727. [ Links ]
31. Liu S, Stampfer M, Hu F et al. Whole grain consumption and risk of coronary heart disease: results from the Nurses´ Health Study. Am J Clin Nutr 1999; 70: 412-419. [ Links ]
32. Wolk A, Manson JE, Stampfer MJ et al. Long-term intake of dietary fiber and decreased risk of coronary heart disease among women. JAMA 1999; 281: 1998-2004. [ Links ]
33. Rouse IL, Armstrong BK, Beilin LJ, Vandongen R. Bloodpressure- lowering effect of a vegetarian diet: Controlled trial in normotensive subjects. Lancet 1983; 321: 5-10. [ Links ]
34. Ródenas, S., Sánchez-Muniz FJ, Bastida, S, Sevillano MI, Larrea Marín T, González-Muñoz MJ. Blood pressure of omnivorous and semi-vegetarian postmenopausal women and their relationship with dietary and hair concentrations of essential and toxic metals. Nutr Hosp 2011; 26: 874-883. [ Links ]
35. Keys A, Grande F, Anderson JT. Fibre and pectin in the diet and serum cholesterol concentration in man. Proc Soc Esp Biol 1961; 106: 555-558. [ Links ]
36. Anderson JW, Allgood LD, Lawrence A, Altringer LA, Jerdack GR, Hengehold DA, Morel JG. Cholesterol-lowering effects of psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: meta-analysis of 8 controlled trials. Am J Clin Nutr 2000; 71: 472-479. [ Links ]
37. Brighenti F. Dietary fructans and serum triacylglycerols: a meta-analysis of randomized controlled trials. J Nutr 2007; 11: 2552S-2556S. [ Links ]
38. Martínez de Prado MT, Sánchez-Muniz FJ, Katan MB, Hermus RJ. The effect of different fiber sources on the neutral steroid excretions of hypercholesterolemic casein fed rabbits. Rev Esp Fisiol 1981; 37: 407-412. [ Links ]
39. Babio N, Balanza R, Basulto J, Bulló M, Salas-Salvadó J. Duetary fibre: influence on body weight, glycemic control and plasma cholesterol profile. Nutr Hosp 2010; 25: 327-340. [ Links ]
40. Topping DL. Propionate as a mediator of the effects of dietary fiber. En Dietary fiber in health and disease. Kritchevsky D, Bonfield C. Eigan Press. Saint Paul, MN; 1995; pp. 340-345. [ Links ]
41. Lairon D. Dietary fibres: effects on lipid metabolism and mechanisms of action. Eur J Clin Nutr 1996; 50: 125-133. [ Links ]
42. Alvarez-Leite JI, Andriex C, Ferezou J, Riottot M, Vieira EC. Evidence for the absence of participation of the microbial flora in the hypocholesterolemic effect of guar gum in gnobiotic rats. Com Biochem Physiol 1994; 109A: 503-510. [ Links ]
43. Zhang JX, Hallmans G, Andersson H, Bosaeus I, Aman P, Tidehag P, Stenling R, Lundin E, Dhlgren S. Effect of oat bran on plasma cholesterol and bile acid excretion in nine subjects with ileostomies. Am J Clin Nutr 1992; 56: 99-105. [ Links ]
44. Nillsson AC, Ostman WM, Knudsen KE, Holst JJ, Riörch IM. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J Nutr 2010; 140: 1932-1936. [ Links ]
45. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999; 69: 30-42. [ Links ]
46. AbuMweis SS, Jew S, Ames NF. beta-glucan from barley and its lipid-lowering capacity: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr 2010; 64: 1472-1480. [ Links ]
47. Lairon D, Bertrais S, Vincent S, Arnault N, Galan P, Boutron MC, Hercberg S; French Supplementation en Vitamines et Mineraux AntioXydants (SU.VI.MAX) Adult Cohort. Dietary fibre intake and clinical indices in the French Supplementation en Vitamines et Mineraux AntioXydants (SU.VI.MAX) adult cohort. Proc Nutr Soc 2003; 62: 11-15. [ Links ]
48. Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault MC. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr 2005; 82: 1185-1194. [ Links ]
49. Van Dam RM, Grievink L, Ocke MC, Feskens EJ. Patterns of food consumption and risk factors for cardiovascular disease in the general Dutch population. Am J Clin Nutr 2003; 77: 1156-1163. [ Links ]
50. Berg CM, Lappas G, Strandhagen E, Wolk A, Toren K, Rosengren A, Aires N, Thelle DS, Lissner L. Food patterns and cardiovascular disease risk factors: the Swedish INTERGENE research program. Am J Clin Nutr 2008; 88: 289-297. [ Links ]
51. Newby PK, Maras J, Bakun P, Muller D, Ferrucci L, Tucker KL. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr 2007; 86: 745-753. [ Links ]
52. Bocanegra A, Bastida S, Benedi J, Nus M, Sanchez-Montero JM, Sanchez-Muniz FJ. Effect of seaweed and cholesterolenriched diets on postprandial lipoproteinaemia in rats. Br J Nutr 2009; 102: 1728-1739. [ Links ]
53. Ren D, Noda H, Amano H, Nishino T, Nishizawa K. Study on antihypertensive and antihyperlipidemic effects of marine algae. Fish Sci 1994; 60: 83-88. [ Links ]
54. Wong KH, Sam SW, Cheung PCK, Ang PO. Changes in lipids profiles of rats fed with seaweed-based diets. Nutr Res 1999; 19: 1519-1527. [ Links ]
55. Olivero-David R, Schultz-Moreira A, Vazquez-Velasco M, Gonzalez-Torres L, Bastida S, Benedi J, Sanchez-Reus MI, Gonzalez-Munoz MJ, Sanchez-Muniz FJ. Effects of Noriand Wakame-enriched meats with or without supplementary cholesterol on arylesterase activity, lipaemia and lipoproteinaemia in growing Wistar rats. Br J Nutr 2011; 106: 1476-1486. [ Links ]
56. Moreira AS, Gonzalez-Torres L, Olivero-David R, Bastida S, Benedi J, Sanchez-Muniz FJ. Wakame and Nori in restructured meats included in cholesterol-enriched diets affect the antioxidant enzyme gene expressions and activities in Wistar rats. Plant Foods Hum Nutr 2010; 65: 290-298. [ Links ]
57. Yang J-L; Kim Y-Hwa; Lee H-S; Lee M-S; Yangha Kim Moon. Barley beta-glucan lowers serum cholesterol based on the Up-regulation of cholesterol 7alpha-hydroxylase activity and mRNA abundance in cholesterol-fed rats. J Nutr Sci Vitaminol 2003; 49: 381-387. [ Links ]
58. Roy S, Freake HC, Fernandez ML. Gender and hormonal status affect the regulation of hepatic cholesterol 7alpha-hydroxylase activity and mRNA abundance by dietary soluble fiber in the guinea pig. Atherosclerosis 2002; 163: 29-37. [ Links ]
59. Kim SC, Seo KK, Kim HW, Lee MY. The effects of isolated lipoproteins and triglyceride, combined oxidized low density lipoprotein (LDL) plus triglyceride, and combined oxidized LDL plus high density lipoprotein on the contractile and relaxation response of rabbit cavernous smooth muscle. Int J Androl 2000; 23 (Suppl. 2): 26-29. [ Links ]
60. Berardi C. Regulacion renal de la tension arterial. Eliminacion de residuos nitrogenados. Insuficiencia renal. En Best & Taylor. Bases fisiologicas de la practica medica. 14 Spanish edition. Dvorkin MD, Cardinali DP, Iermoli RH. Panamericana. Buenos Aires; 2010; pp. 541-554. [ Links ]
61. González-Muñoz MJ, Sánchez-Muniz FJ, Ródenas S, Sevillano MI, Larrea Marín MT, Bastida S. Differences in metal and metalloid content in the hair of normo- and hypertensive postmenopausal women. Hypertens Res 2010; 33: 219-224. [ Links ]
62. Streppel MT, Arends LR, van 't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005; 165: 150-156. [ Links ]
63. Arvill A, Bodin L. Effect of short-term ingestion of konjac glucomannan on serum cholesterol in healthy men. Am J Clin Nutr 1995; 61: 585-589. [ Links ]
64. Birketvedt GS, Asseth J, Florholmen JR, Ryttig K. Long-term effect of fibre supplement and reduced energy intake on body weight and blood lipids in overweight subjects. Acta Medica (Hradec Kralove) 2000; 43: 129-132. [ Links ]
65. Brussaard JH, Raaij JM van, Stasse-Wolthuis M, Katan MB, Hautvast JG. Blood pressure and diet in normotensive volunteers: absence of an effect of dietary fiber, protein, or fat. Am J Clin Nutr 1981; 34: 2023-2029. [ Links ]
66. Burke V, Hodgson JM, Beilin LJ, Giangiulioi N, Rogers P, Puddey IB. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension 2001; 38: 821-826. [ Links ]
67. Eliasson K, Ryttig KR, Hylander B, Rossner S. A dietary fibre supplement in the treatment of mild hypertension: a randomized, double blind, placebo controlled trail. J Hypertens 1992; 10: 195-199. [ Links ]
68. Fehily AM, Burr ML, Butland BK, Eastham RD. A randomised controlled trial to investigate the effect of a high fibre diet on blood pressure and plasma fibrinogen. J Epidemiol Community Health 1986; 40: 334-337. [ Links ]
69. Hagander B, Asp NG, Ekman R, Nilsson-Ehle P, Lundquist I, Schersten B. Dietary fibre enrichment, blood pressure, lipoprotein profile and gut hormones in NIDDM patients. Eur J Clin Nutr 1989; 43: 35-44. [ Links ]
70. He J, Streiffer RH, Muntner P, Krousel-Wood MA, Whelton PK. Effect of dietary fiber intake on blood pressure: a randomized, double-blind, placebo-controlled trial. J Hypertens 2004; 22: 73-80. [ Links ]
71. Keenan JM, Pins JJ, Frazel C, Moran A, Turnquist L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: a pilot trial. J Fam Pract 2002; 51: 369. [ Links ]
72. Little P, Girling G, Hasler A, Trafford A, Craven A. A controlled trial of a low sodium, low fat, high fibre diet in treated hypertensive patients: the efficacy of multiple dietary intervention. Postgrad Med J 1990; 66: 616-621. [ Links ]
73. Margetts BM, Beilin LJ, Vandongen R, Armstrong BK. A randomized controlled trial of the effect of dietary fibre on blood pressure. Clin Sci (Lond) 1987; 72: 343-350. [ Links ]
74. Nami R, Gallo V, Pavese G, Panza F, Gennari C. Antihypertensive activity of a vegetable fibre preparation: a preliminary, double-blind, placebo controlled study. Eur J Clin Nutr 1995; 49 (Suppl. 1): S201-S206. [ Links ]
75. Önning G, Wallmark A, Persson M, Akkeson B, Elmstahl S, Oste R. Consumption of oat milk for 5 weeks lowers serum cholesterol and LDL cholesterol in free-living men with moderate hypercholesterolemia. Ann Nutr Metab 1999; 43: 301-309. [ Links ]
76. Rigaud D, Ryttig KR, Angel LA, Apfelbaum M. Overweight treated with energy restriction and a dietary fibre supplement: a 6-month randomized double-blind, placebo-controlled trial. Int J Obes 1990; 14: 763-769. [ Links ]
77. Rössner S, von Zweigbergk D, Ohlin A, Ryttig K. Weight reduction with dietary fibre supplements: results of two doubleblind randomized studies. Acta Med Scand 1987; 222: 83-88. [ Links ]
78. Rössner S, Andersson IL, Ryttig K. Effects of a dietary fibre supplement to a weight reduction programme on blood pressure: a randomized, double blind placebo-controlled study. Acta Med Scand 1988; 223: 353-357. [ Links ]
79. Ryttig KR, Tellnes G, Haegh L, Boe E, Fagerthun H. A dietary fibre supplement and weight maintenance after weight reduction: a randomized double-blind, placebo-controlled long-term trial. Int J Obes 1989; 13: 165-171. [ Links ]
80. Ryttig KR, Lammert O, Nielsen E, Garby L, Poulsen K. The effect of a soluble dietary fibre supplement on 24-hour energy expenditure during a standardized physical activity programme. Int J Obes 1990; 14: 451-455. [ Links ]
81. Schlamowitz P, Halberg T, Warnoe O, Wilstrup F, Ryttig K. Treatment of mild to moderate hypertension with dietary fibre. Lancet 1987; 2: 622-623. [ Links ]
82. Solum TT, Ryttig KR, Solum E, Larsen S. The influence of a high-fibre diet on body weight, serum lipids and blood pressure in slightly overweight persons: a randomized, double-blind, placebo-controlled investigation with diet and fiber tablets (Dumo Vital). Int J Obes 1987; 11 (Suppl. 1): 67-71. [ Links ]
83. Swain JF, Rouse IL, Curley CB, Sacks FM. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N Engl J Med 1990; 322: 147- 152. [ Links ]
84. Törrönen R, Kansanen L, Uusitupa M, et al. Effects of an oat bran concentrate on serum lipids in free-living men with mild to moderate hypercholesterolaemia. Eur J Clin Nutr 1992; 46: 621-627. [ Links ]
85. Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens 2005; 23: 475-481. [ Links ]
86. Van Horn L, Moag-Stahlberg A, Liu KA et al. Effects on serum lipids of adjusting instant oats to usual American diets. Am J Public Health 1991; 81: 183-188. [ Links ]
87. Jenkins DJA, Kendall CWC, Popovich DG, Vidgen E, Mehling CC et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism 2001; 50: 494-503. [ Links ]
88. Jenkins DJA, Kendall CWC, Vuksan V, Vidgen E, Parker T, Faulkner D, et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am J Clin Nutr 2002; 75: 834-839. [ Links ]
89. Kelsay JL, Behall KM, Prather ES. Effect of fiber from fruits and vegetables on metabolic responses of human subjects I. Bowel transit time, number of defecations, fecal weight, urinary excretions of energy and nitrogen and apparent digestibilities of energy, nitrogen, and fat. Am J Clin Nutr 1978; 31: 1149-1153. [ Links ]
90. Pins JJ, Geleva D, Keenan JM, Frazel C, O´Connor PJ, Cherney LM. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? J Fam Pr 2002; 51: 353-359. [ Links ]
91. Saltzman E, Das SK, Lichtenstein AH, Dallal GE, Corrales A, Schaefer EJ et al. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. J Nutr 2001; 131: 1465-1470. [ Links ]
92. Singh RB, Rastogi SS, Singh R, Ghosh S, Niaz MA. Effects of guava intake on serum total and high-density lipoprotein cholesterol levels and on systemic blood pressure. Am J Cardiol 1992; 70: 1287-1291. [ Links ]
93. Uusitupa M, Siitonen O, Savolainen K, Silvasti M, Penttila I, Parviainen M. Metabolic and nutritional effects of long-term use of guar gum in the treatment of noninsulin-dependent diabetes of poor metabolic control. Am J Clin Nutr 1989; 49: 345- 351. [ Links ]
94. Maki KC, Galant R, Samuel P, Tesser J, Witchaer MS, Ribaya-Merado JD, Blumberg JB, Geohas J. Effects of consuming foods containing oat beta-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. Eur J Clin Nutr 2007; 61: 786-795. [ Links ]
95. Lecumberri E, Mateos R, Ramos S, Alía M, Rúperez P, Goya L, Izquierdo-Pulido M, Bravo L. Characterization of cocoa fiber and its effect on the antioxidant capacity of serum in rats. Nutr Hosp 2006; 21: 622-628. [ Links ]
96. Anderson JW, Hanna TJ, Peng X, Kryscio RJ. Whole grain foods and heart disease risk. J Am Coll Nutr 2000; 19 (3 Suppl.): 291S-299S. [ Links ]
97. Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willet WC. Vitamin E consumption and the risk of coronary disease in women. N Eng J Med 1993; 328: 1444-1449. [ Links ]
98. Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Golditz GA, Willet WC. Vitamin E consumption and the risk of coronary disease in men. N Eng J Med 1993; 328: 1450-1460. [ Links ]
99. Gómez-Juristi M, González-Torres L, Bravo L, Vaquero MP, Bastida S, Sánchez-Muniz FJ. Efectos beneficiosos del chocolate en la salud cardiovascular. Nutr Hosp 2011; 26: 287-290. [ Links ]
100. Nus M, Sánchez-Muniz FJ, Sánchez-Montero JM. A new method for the determination of arylesterase activity in human serum using simulated body fluid. Atherosclerosis 2006; 188: 155-159. [ Links ]
101. Nus M, Frances F, Librelotto J, Canales A, Corella D, Sánchez- Montero JM, Sánchez-Muniz FJ. Arylesterase activity and antioxidant status depend on PON1-Q192R and PON1-L55M polymorphisms in subjects with increased risk of cardiovascular disease consuming walnut-enriched meat. J Nutr 2007; 137: 1783-1788. [ Links ]
102. Asp N-G., Contor L. Process for the assessment of scientific support for claims on foods (PASSCLAIM): overall introduction. Eur J Nutr 2003; 42 (Suppl. 1): 3-5. [ Links ]
103. Oliveira A, Rodríguez-Artalejo F, Gaio R, Santos AC, Ramos E, Lopes C. Major habitual dietary patterns are associated with acute myocardial infarction and cardiovascular risk markers in a southern European population. J Am Diet Assoc 2011; 111: 241-250. [ Links ]
104. Librelotto J, Bastida S, Serrano A, Cofrades S, Jiménez-Colmenero F, Sánchez-Muniz FJ. Changes in fatty acids and polar material of restructured low-fat or walnut-added steaks pan-fried in olive oil. Meat Sci 2008; 80: 431-441. [ Links ]
105. Jiménez-Colmenero F, Sánchez-Muniz FJ, Olmedilla-Alonso B, and Collaborators: Ayo J, Carballo J, Cofrades S, Ruiz-Capillas C, Serrano A, Bastida S, Benedí J, Canales A, Librelotto J, Nus M, Blanco-Navarro I, Blázquez-García S, Granado-Lorencio F, Herrero-Barbudo C. Design and development of meat-based functional foods with walnut: Technological, nutritional and health impact. Food Chem 2010; 123: 959-967. [ Links ]
106. Canales A, Bastida S, Librelotto J, Nus M, Benedí J, Sánchez-Muniz FJ. Platelet aggregation, eicosanoid production and thrombogenic ratio in individuals at high risk consuming walnut-enriched meat. A cross-over, placebo-controlled study. Br J Nutr 2009; 102: 134-141. [ Links ]
107. Canales A, Benedi J, Bastida S, Corella D, Guillen M, Librelotto J, Nus M, Sánchez-Muniz FJ. The effect of consuming meat enriched in walnut paste on platelet aggregation and thrombogenesis varies in volunteers with different apolipoprotein A4 genotype. Nutr Hosp 2010; 2: 746-754. [ Links ]
108. Canales A, Sánchez-Muniz FJ, Bastida S, ibrelotto J, Nus M, Corella MD,Guillén M, Benedí J. Effect of walnut-enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur J Clin Nutr 2011; 65: 703-710. [ Links ]
109. López-López I, Bastida S, Ruiz-Capillas C, Bravo L, Larrea MT, Sánchez-Muniz F, Cofrades S, Jiménez-Colmenero F. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci 2009; 83: 492-498. [ Links ]
110. Schutz Moreira A, Benedí J, González-Torres L, Olivero-David R, Bastida S, Sánchez-Reus MI, González-Muñoz MJ, Sánchez-Muniz FJ. Effects of diet enriched with restructured meats containing Himanthalia elongate, on hypercholesterolaemic induction, CYP7A1 expression and antioxidant enzyme activity and expression in growing rats. Food Chem 2011; 129: 1623-1630. [ Links ]
111. Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 2006; 112: 71-105. [ Links ]
112. Sánchez-Muniz FJ, Bastida S, Gutiérrez-García O, Carbajal A. Olive oil-diet improves the simvastatin effects with respect to sunflower oil-diet in men with increased cardiovascular risk: a preliminary study. Nutr Hosp 2009; 24: 333-339. [ Links ]
113. Vaquero MP, Sánchez Muniz FJ, Jiménez Redondo S, Prats Oliván P, Higueras FJ, Bastida S. Major diet-drug interactions affecting the kinetic characteristics and hypolipidaemic properties of statins. Nutr Hosp 2010; 25: 193-206. [ Links ]
114. Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statinassociated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27: 146-151. [ Links ]
115. Villalobos ME, Sánchez-Muniz FJ, Acín MT, Vaquero MP, Higueras FJ, Bastida S. Similarities, differences and agonisms of pleiotropic effects of statins and omega-3 fatty acids. Nutr Hosp 2010; 25: 889-909. [ Links ]
116. Moreyra AE, Wilson AC, Koraym A. Effect of combining psyllium fiber with simvastatin in lowering cholesterol. Arch Intern Med 2005; 165: 1161-1166. [ Links ]
117. Arbonés G, Carbajal A, Gonzalvo B, González-Gross M, Joyanes M, Marques-Lopes I, Martín ML, Martínez A, Montero P, Núñez C, Puigdueta I, Quer J, Rivero M, Roset MA, Sánchez-Muniz FJ, Vaquero MP. Nutrition and dietary recommendations for the elderly "Public Health" Working Group of the Spanish Nutrition Society. Nutr Hosp 2003; 18: 109-137. [ Links ]
118. Food and Nutrition Board (FNB), Institute of Medicine (IONM). Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and aminoacids (Macronutrients). 2002. [ Links ]
119. Federación Española de Sociedades de Nutrición, Alimentación y Dietética (FESNAD). Ingestas dietéticas de referencia para la población Española. EUNSA Ed. SA. Universidad de Navarra. Pamplona. 2010. [ Links ]
120. Mann J. Dietary carbohydrate: relationship to cardiovascular disease and disorders of carbohydrate metabolism. Eur J Clin Nutr 2007; 61: S100-S111. [ Links ]
121. Hardin-Fanning F. The effects of a Mediterranean-style dietary pattern on cardiovascular disease risk. Nurs Clin North Am 2008; 43: 105-115. [ Links ]
122. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009; 119: 1093-1100. [ Links ]
123. Ordovas JM. The genetic of serum responsiveness to dietary interventions. Proc Nutr Soc 1999; 58: 171-187. [ Links ]
124. Ordovas JM. Nutrigenetics, plasma lipids, and cardiovascular risk. J Am Diet Assoc 2006; 106: 1074-1081. [ Links ]
125. Fundación Faustino Orbegozo Eizaguirre. Curvas y tablas de crecimiento (Estudio longitudinal y transversal). Instituto de Investigación sobre crecimiento y desarrollo. Bilbao. http://www.aepap.org/crecorbegozo.htm [ Links ]
126. American Academy of Pediatrics. Commitee on Nutrition. Cholesterol in Chilhood. Pediatrics 1998; 101: 141-147. [ Links ]
127. Ecija Peiró JL, Vazquez Martul M. Hipertensión arterial. En: Pediatría extrahospitalaria. Ergón. Madrid, 2001. [ Links ]
![]() Correspondence:
Correspondence:
Francisco José Sánchez-Muniz.
Departamento de Nutrición y Bromatología I (Nutrición).
Facultad de Farmacia.
Plaza Ramón y Cajal, s/n. 28040 Madrid.
E-mail: frasan@farm.ucm.es
Recibido: 22-VI-2011.
1.a Revisión: 14-X-2011.
Aceptado: 14-X-2011.