My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.27 n.4 Madrid Jul./Aug. 2012
https://dx.doi.org/10.3305/nh.2012.27.4.5869
ORIGINAL
Nutritional status and adequacy of enteral nutrition in pediatric cancer patients at a reference center in northeastern Brazil
El estado nutricional y la adecuación de la nutrición enteral en pacientes de oncología pediátrica en un centro de referencia del noreste de Brasil
J. Maciel Barbosa1, F. Pedrosa2 and P. Coelho Cabral3
1Msc in Nutrition. Federal University of Pernambuco (UFPE). Nutricionist in the Departament of Nutrition. Institute of Integrative Medicine Professor Fernando Figueira. IMIP.
2Specialisty in Paediatric Oncology by the Brazilian Medical Association. Medical Director of the Pediatric Oncology Unit. IMIP.
3PhD in Nutrition. UFPE. Professor at the Departament of Nutrition. UFPE. Recife. Brazil.
The Research and Teaching Support Fund (FAPE/ IMIP) offered funding for the publication of this paper.
ABSTRACT
Introduction: Individualized nutritional support is important to pediatric cancer patients and should be integrated to the overall treatment of these patients.
Objective: Analyze the nutritional status of cancer patients submitted to enteral nutrition (EN) and assess the adequacy of this form of nutrition.
Methods: A case series study was carried out at the Pediatric Oncology Unit of the Institute of Integrative Medicine Professor Fernando Figueira (IMIP, Brazil, Recife-PE) between January and December 2009. Clinical and anthropometric data were obtained from medical charts and nutritional follow-up charts. Z scores for height for age, weight for age and body mass index for age indicators (H/A, W/A and BMI/A, respectively) were calculated using the AnthroPlus® program. Caloric and protein requirements were calculated based on the recommendations of the Brazilian National Council of Oncologic Nutrition.
Results: At the beginning of EN, 32.4% of the sample had short stature and 23.9% were underweight based on the BMI/A indicator. The assessment of EN adequacy demonstrated that 49.3% reached the caloric requirements and 76.1% reached the protein requirements, with maximal intakes of 65.6 Kcal/Kg/day and 1.95 g of protein/kg/day. Malnourished patients had greater mean Z scores for W/A and BMI/A at the end of EN, whereas no significant changes were found among patients with adequate nutritional status and significant reductions in these indicators were found among those with overweight or obesity.
Conclusion: The patients either maintained or achieved a significant improvement in nutritional status, which demonstrates the importance of nutritional support and follow up during hospitalization.
Key words: Nutritional status. Enteral nutrition. Tube feeding. Cancer. Children.
RESUMEN
Introducción: El soporte nutricional individualizado se considera una terapia importante en oncología pediátrica y, como tal, debe integrarse en el tratamiento global de estos pacientes.
Objetivo: Evaluar el estado nutricional de los pacientes sometidos a terapia de la nutrición enteral y la adecuación de la nutrición enteral (NE).
Métodos: Una serie de casos realizado en la Oncología Pediátrica del Instituto de Medicina Integrativa Profesor Fernando Figueira (IMIP, Brasil, Recife-PE), entre los meses de enero a diciembre de 2009. Se obtuvieron los datos antropométricos y clínicos de los registros médicos y formularios para recibir asesoramiento nutricional. Los valores de puntuación Z de los indicadores de A/E, P/E y el IMC/E se calcularon utilizando el software AnthroPlus®. Las necesidades calóricas y proteínas se calcularon de acuerdo con el Consenso Nacional de Nutrición Oncológica.
Resultados: Al inicio de la terapia se obtuvo 32,4% de retraso del crecimiento y 23,9% de bajo peso según el IMC/E. La evaluación de la adecuación de NE mostró que el 49,3% llegó a las necesidades calóricas y 76,1% las necesidades de proteínas, cuya contribución máxima fue de 65,6 kcal/kg/día y 1,95 g/kg/día de proteína. Los pacientes desnutridos tienen mayor media de las puntuaciones para Z de P /E y IMC/E al final de la NE. Los pacientes eutróficos tuvieron ningún cambio significativo, mientras aquellos con el sobrepeso o obesos tuvieron una reducción significativa. El grupo de desnutridos tuvieron una mayor ingesta media de calorías y proteínas durante el NE.
Conclusión: Los pacientes tuvieron mantenimiento o una mejoría significativa del estado nutricional, poniendo de relieve la necesidad de asesoramiento nutricional durante la hospitalización.
Palabras clave: Estado nutricional. Nutrición enteral. Alimentación por sonda. Cáncer. Niños.
Abbreviations
ASPEN: American Society for Parenteral and Enteral Nutrition.
BMI/A: Body mass index-for-age.
DRI: Dietary Reference Intake.
EN: Enteral nutrition.
H/A: Height-for-age.
IMIP: Institute of Integrative Medicine Professor Fernando Figueira.
IQ: Interquartile.
NCON: National Council of Oncologic Nutrition.
PN: Parenteral nutrition.
W/A: Weight-for-age.
WHO: World Health Organization.
Introduction
Malnutrition is frequent among children with cancer and may be present at the time of diagnosis or may emerge during treatment. The frequency upon diagnosis ranges from 6 to 50%, depending on the type, location, degree of malignancy and stage of the tumor as well as the criteria used for the nutritional assessment.1 Malnutrition may be caused by a number of mechanisms involving the tumor itself and the host response to both the tumor and treatment. Malnutrition is often associated with a greater risk of infection, a lesser response to treatment, greater toxicity stemming from chemotherapy and lower survival rate.1,2 Individualized nutritional support is important to pediatric cancer patients and should be integrated to the overall treatment of these patients.3
In recent decades, enteral nutrition (EN), also known as tube feeding, has been widely used due to its numerous benefits, such as a lesser risk of infection and other complications related to the catheter in comparison to parenteral nutrition.1,3-5 Moreover, EN is less expensive, more physiological and maintains the integrity of the intestinal mucosa, thereby reducing the risk of bacterial translocation.4,5 Technological advances in the implementation of EN and the development of increasingly specialized dietary formulas have contributed to the growing use of EN therapy.6,7
EN is indicated for all patients who cannot, should not or do not want to eat through the oral pathway and have a functioning gastrointestinal tract, which is essential to the use of this type of nutritional therapy.7 Total parenteral nutrition should be reserved for cases in which the total or partial use of the gastrointestinal tract is impossible or access is hindered due to thrombocytopenia.3-5
There are few well-controlled studies assessing the risks and benefits of EN therapy among pediatric cancer patients. Moreover, EN is suggested in the following situations: when nutrition through the oral pathway is not possible; when food intake falls short of nutritional requirements; and in the presence of nutritional risk or malnutrition.3,8
Objectives
The aims of the present study were to analyze the nutritional status of children and adolescents with cancer submitted to enteral nutrition, assess the adequacy of this form of nutrition and determine the frequency of complications associated to this therapy.
Methods
Study design and site
A case series study was carried out between January and December 2009, involving male and female children and adolescents with a diagnosis of cancer hospitalized at the Pediatric Oncology Unit of the Institute of Integrative Medicine Professor Fernando Figueira (IMIP), in the city of Recife (northeastern Brazil) for cancer treatment or the treatment of complications stemming from this disease.
Study population
All pediatric patients hospitalized with cancer and who received exclusive EN or mixed EN (in combination with an oral diet or parenteral nutrition) for a period of 24 hours or more throughout the study period were included. At IMIP, all patients submitted to EN therapy receive industrialized enteral formulas with infusion in an open system.
Data acquisition
Secondary data was obtained from the EN follow up charts used by the IMIP Department of Nutrition as well as the electronic medical chart of each patient. On the nutrition charts, information is recorded daily on age, type of neoplasm and EN follow up data, such as access pathway, volume, type of diet, calories and protein offered, complications secondary to EN as well as weight and height measurements at the beginning and end of EN therapy. From the patient medical charts, information was collected on the indication for EN and conditions at the end of therapy and discharge from hospital (oral nutrition, enteral nutrition or death).
Anthropometrics
Anthropometric measures were obtained based on the protocol of the IMIP Pediatric Oncology Unit. For children under two years of age, weight was determined on an electronic scale (Kratos®) with a precision of 0.1 kg and capacity of 15 kg and height was determined in the horizontal position using an infantometer with a maximal length of 1.20 m and a precision of 0.1 cm. For children over two years of age, weight was determined on a digital scale (Filizola®) with a precision of 0.5 kg and a capacity of 150 kg and height was determined using a stadiometer coupled to the scale.
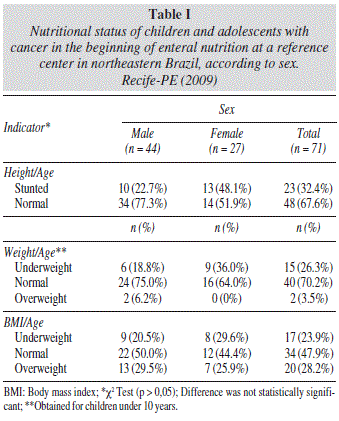
Nutritional diagnosis was determined using height for age (H/A), weight for age (W/A) and body mass index for age (BMI/A) indicators according to gender, using the reference standards recommended by the World Health Organization (WHO) and employing the WHO AnthroPlus® program (WHO, Geneva, Switzerland).9,10 The W/A indicator was calculated only for children under 10 years of age. The results were expressed in Z scores, considering children and adolescents with scores below two standard deviations (SD) as having nutritional deficit, those with greater than one SD for the BMI/A indictor as having overweight/ obesity and those with more than two SD for the W/A indicator as having excessive weight.11
Nutritional requirements
The reference used for the nutritional requirements was the Brazilian National Council of Oncologic Nutrition, done by National Institute of Cancer (INCA, Brazil).7 The caloric requirements were calculated with equations proposed by the Dietary Reference Intake7,12 and protein requirements were calculated based on the recommendations of the American Society of Parenteral and Enteral Nutrition (ASPEN). 7,13 For these calculations, age in years, weight in kilograms and height in meters were considered at the beginning and end of EN therapy. Ideal weight was used for those with malnutrition (Z score for BMI/A < -2SD) and was defined as the weight of the 50th percentile for the BMI/A indicator, provided this value did not exceed 20% of actual weight.7 A physical activity coefficient of 1.0 was also considered for these calculations, as the sample was made up of hospitalized patients. The calculation of the caloric value did not include the glycosylated serum infused with medications.
Data analysis
The adequacy of the offer (calories and protein) was calculated by comparing the values obtained from the percentage relationship between the offer and recommended requirements, and classified as underfeeding (< 90% of required), adequate feeding (90% to 110% of required), or overfeeding (> 110% of required).14,15 Maximal adequacy in the period was also evaluated (day of greatest offer of calories and protein). The duration of therapy was obtained in days beginning with the first day of EN therapy until its complete discontinuance.
Statistical analysis
The data were entered into the Excel program for Windows®. Analyses were performed using the SPSS program (version 13.0). Continuous variables were tested for normality using the Kolmogorov-Smirnov test. ANOVA was used to compare more than two independent groups for data with normal distribution and the Kruskal-Wallis test was used for data with non-normal distribution. The paired Student's t-test was used for the comparison of means for the dependent variables. The chi-square (χ2) test was used for the comparison of proportions. The level of significance was set to 5% (p < 0.05)
Ethical considerations
This study received approval from the IMIP Bioethics Committee under process n.o1764 and all procedures were carried out in compliance with the ethical standards of the committee responsible for experiments involving human subjects.
Results
A total of 2,927 hospitalizations occurred in the study period, with 71 episodes of EN, corresponding to 2.4% of the total number of hospitalizations. Twelve patients underwent EN in two or more separate hospitalizations, which were considered distinct episodes. Due to cancer treatment, children and adolescents often need hospitalization for the specific treatment of infection, symptoms, hydroelectrolytic disorders, surgery, etc., meaning that the number of hospitalizations does not equal the number of patients at a pediatric oncology unit.
Patient age ranged from one month to 18 years, with a predominance of children under five years of age (56.3%). Males accounted for 62.0% of the sample and the most prevalence neoplasms were solid tumors, accounting for 57.8% of the total, among which 29.6% were tumors of the central nervous system, 11.3% were neuroblastomas and 8.5% were rhabdomyosarcomas.
Table I displays the results of the assessment of nutritional status at the beginning of EN therapy according to gender based on the H/A, W/A and BMI/A indicators. In the overall sample, 32.4% had a short stature for age and 26.3% were underweight based on the W/A indicator, whereas 23.9% were classified as underweight and 28.2% were classified with overweight/obesity based on the BMI/A indicator.
The main indications for EN therapy were intensive care with mechanical ventilation (31.0%), neurological impairment secondary to the base disease (central nervous system neoplasm or encephalopathy) (21.1%); anorexia (15.5%); and malnutrition/weight loss (15.5%).
Table II describes the main variables related to EN. The evaluation of the calorie and protein offer demonstrates that less than half of the sample (49.3%) reached the caloric requirements and 78.9% reached the protein requirements, with maximal intakes of 65.6 kcal/kg/ day and 1.95 g of protein/kg/day. The median duration of therapy was seven days (interquartile [IQ] interval: 3-11). Regarding the type of nutritional therapy, 80.3% of the patients received exclusive EN and 19.7% received mixed EN (combined with oral or parenteral nutrition). EN access in most cases occurred though a nasogastric tube (90.1%). The predominant diet was an industrialized polymeric formula adjusted to age (88.7%), followed by an oligomeric formula (11.3%). At the end of nutritional therapy, 54.9% of the patients were discharged from hospital with oral nutrition and 16.9% were discharged with at-home enteral nutrition, whereas 28.2% died stemming from complications related to the neoplasm.
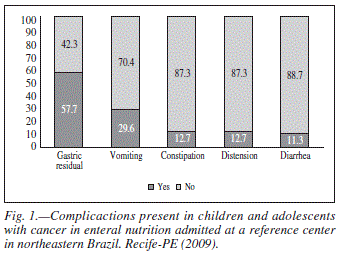
Throughout EN therapy, changed occurred in the characteristics of the diets prescribed due to the clinical condition of the patient and/or gastrointestinal intolerance to the formula initially offered. Such changes included a change from a polymeric diet to an oligomeric diet (11.3% of the patient), lactose restriction in relation to the initial diet (9.9%), a reduction in the dilution of the initial formula (16.9%), a reduction in the volume during EN (35.2%) and the discontinuation of the diet due to clinical complications or complications secondary to nutritional therapy (26.8%). Figure 1 lists the main complications associated with the use of EN in the sample, highlighting gastric residual volume (57.7%) and vomiting (29.6%).
Table III displays the mean Z scores of the anthropometric indicators at the beginning and end of EN therapy. Malnourished patients had significantly greater mean Z scores for W/A and BMI/A at the end of EN therapy (p> 0.05), whereas no significant changes in the Z scores for these indicators were found among patients with adequate nutritional status and significant reductions in these indicators were found among those with overweight or obesity. Moreover, the malnourished group had the highest mean calorie and protein intake values throughout EN, with statistically significant differences (p < 0.05) (table IV).
Discussion
In the present study, EN was only employed in 2.4% of the 2,927 hospitalizations in the period analyzed. Studies on the frequency of EN use in pediatric patients, especially cancer patients, are scarce. Oliveira et al.16 report that only 3.2% of patients hospitalized in a pediatric infection infirmary received EN therapy.
The anthropometric indicators revealed important nutritional deficit, as the frequencies for all indicators used in the present study surpassed WHO reference values, in which only 2.3% of the general population is expected to have Z scores below -2SD.17 However, the comparison of the results with other studies is limited due to the use of different indicators, cutoff points and reference curves as well as different study population characteristics, such as the type of neoplasm and phase of treatment.
In a study carried out involving pediatric patients with cancer at the beginning of treatment, Araûjo et al.18 found lower frequencies of nutritional deficit. The H/A and W/A indices revealed that 10% of the sample was short for their age and 3.3% were underweight for their age. The BMI/A index revealed that 13.3% were underweight and 23.6% were overweight. Zalina et al.19 found greater frequencies of nutritional deficit in a study involving children with leukemia between four and 12 years of age, with 37.3% and 31.4% of the 51 patients considered malnourished by the weight for age and weight for height indicators, respectively, and 17.6% had a short stature for age.
Regarding the effect of nutritional deficit on stature, nearly one third of the sample analyzed (32.4%) had Z scores below -2SD at the beginning of nutritional support regarding the H/A indicator. Similarly, Sarni et al.20 report short stature for age in 30% of children under five years of age hospitalized in general pediatric infirmaries in a study involving 10 university or teaching hospitals in the northeastern, southeastern, central western and southern regions of Brazil. Even more alarming figures are reported in a study involving pediatric cancer patients admitted to a reference hospital in the east African country of Malawi, with a stature deficit frequency of 44.5%.21
A number of factors affect linear growth during cancer treatment, including the disease itself, inadequate nutrition, the occurrence of infections, chemotherapy and growth hormone deficiency secondary to cranial radiation.22 The sum of all these factors determines a deceleration of growth during treatment for cancer23 and may be related to the frequency of short stature in the present study.
The majority of indications for EN were related to conditions that render oral feeding impossible, such as the use of mechanical ventilation (31%) and neurologic impairment (21.1%). In patients with cancer, indications for EN are generally more related to situations that limit food intake and/or denote nutritional risk (malnutrition, weight loss, anorexia, mucositis, etc.),24,25 but EN may also be indicated in cases of neurologic impairment and/or a high risk of bronchoaspiration.26 Thus, one cannot discard the possibility that the frequency of EN was below the recommended use, as this aspect was not evaluated in the present study.
There was a low percentage of hyperalimentation, but a greater frequency of patients who did not achieve their estimated nutritional requirements. This finding may be explained by the severe clinical conditions of the patients and occurrence of gastrointestinal complications, which imply restrictions regarding volume and the offer of macronutrients. Moreover, there was a 19.7% frequency of the concomitant use of oral and/or parenteral nutrition, the caloric and protein values of which were not included in the calculation of the total offer.
The most frequent clinical complications associated with EN therapy were gastric residual volume and vomiting. In a study involving children with cancer in EN therapy, Pietsch et al.27 report that vomiting occurred in the majority of the sample (71%), but was likely associated with chemotherapy and generally persisted even after a pause in the infusion of the diet.
Cancer patients often experience complications that affect the gastrointestinal tract, which may be associated with the direct toxicity of treatment and/or an aggravation of the clinical condition. Gastric residual volume is the most frequent complication in critical patients under gastric EN therapy. The cause is multi-factor, but appears to be related to the use of drugs that diminish gastric motility as well as alterations in gastric emptying.28 Vomiting has been associated with gastro-esophageal reflux, which may be related to postural factors, the caliber of the tube and dysfunction of the lower esophageal sphincter.29 In such situations, the use of prokinetic agents and a transpyloric feeding tube is recommended to minimize the risk of bronchoaspiration.28
The median duration of EN was less than one week (median: 7 days, IQ: 3 to 11), which is considered less time than necessary for an effect on nutritional status. In a study involving pediatric patients in EN therapy, Ezge et al.30 found a greater median value for the duration of therapy (median: 21.5 days; IQ: 2 to 547) and higher mean Z score at the end of EN for the W/A, H/A and weight for height indicators. In a review on the impact of nutritional therapy among cancer patients, Bozzetti31 concluded that the improvement in nutritional status in the studies analyzed was likely limited due to the fact that the duration of therapy was less than a few weeks, whereas the development of malnutrition in cancer often occurs over the course of months. The author also suggests that metabolic abnormalities present in cancer may limit the efficacy of nutritional support.
The early indication of EN therapy is currently one of the main goals of nutritional therapy in children and adolescents with cancer.32 A number of studies have demonstrated that EN should be initiated within the first 24 to 48 hours following trauma, surgery or hospitalization, the benefits of which are the attenuation of the metabolic response, maintenance of functional and structural integrity of the intestinal mucosa, with a reduction in its permeability and the translocation of bacteria and endotoxins, a reduction in the impairment of the immune response, etc., with a consequent reduction in hospitalization time and costs.33,34 No data on the time elapsed between hospitalization and the onset of EN were collected in the present study and it is therefore not possible to analyze the adequacy of the timely establishment of this therapy.
The malnourished children had a significant improvement in Z scores for the W/A and BMI/A indicators during the use of EN. These findings may be explained by the greater caloric and protein intake among these patients. However, no statistically significant differences were found regarding the duration of therapy and the time needed to reach nutritional requirements among the groups analyzed. Among the patients with adequate nutrition, no difference occurred in mean Z scores for the W/A and BMI/A, indicating that no nutritional impairment had occurred during hospitalization. The children with overweight or obesity at the onset of EN had a significant reduction in Z scores for these same indicators. While cancer treatment is not the best time for weight loss, this finding may be explained by the replacement of high-calorie foods and sweets with adequate nutritional intake during hospitalization.
EN therapy has limitations in cancer patients due to therapeutic complications secondary to the toxicity of treatment (nausea, vomiting and diarrhea). However, the patients submitted to EN therapy in the present study either maintained or achieved a significant improvement in nutritional status, which demonstrates the importance of nutritional support and follow up during hospitalization. Moreover, a multidisciplinary team is important for the adequate support to children and adolescents with cancer in EN therapy.
References
1. Ladas EJ, Sacks N, Meacham L, Henry D, Enriquez L, Lowry G, Hawkes R, Dadd G, Rogers P. A Multidisciplinary Review of Nutrition Considerations in the Pediatric Oncology Population: A Perspective From Children's Oncology Group. Nutr Clin Pract 2005; 20 (4): 377-393. [ Links ]
2. Van CE, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 2005; 9 (Suppl. 2): S51-63. [ Links ]
3. Brasil. Ministério da Saúde. Instituto Nacional de Câncer. Consenso Nacional de Nutrição Oncológica. Rio de Janeiro: INCA, 2009. [ Links ]
4. Sala A, Wade L, Barr RD. Nutritional support for children with cancer. Indian J Pediatri 2003; 70: 813-816. [ Links ]
5. Bankhead R, Boullata J, Brantley S et al. ASPEN. Enteral Nutrition Practice Recommendations. JPEN 2009; 33; 122-167. [ Links ]
6. Campos DJ, Silva AFF, Souza MH et al. Otimização do fornecimento calórico-protéico na terapia nutricional enteral em unidade de terapia intensiva com uso de protocolo. Rev Bras Nutr Clin 2006; 21 (1): 2-5. [ Links ]
7. Hernández JA, Torres NP, Jiménez AM. Utilización clínica de la Nutrición Enteral. Nutr Hosp 2006; 21 (Suppl. 2): 87-99. [ Links ]
8. Bozzetti F, Mori V. Nutritional support and tumour growth in humans: a narrative review of the literature. Clin Nutr 2009; 28:226-30. [ Links ]
9. WHO Multicentre Growth Reference Study Group: WHO Child Growth Standards: Length/height-for-age, weight-forage, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva, World Health Organization, 2006. Available at: http://www.who.int/childgrowth/standards/technical_report/en/index.html [ Links ]
10. WHO Anthro for personal computers, version 3.1, 2010: Software for assessing growth and development of the world's children. Geneva: WHO, 2010 (http://www.who.int/childgrowth/software/en/). [ Links ]
11. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Protocolos do Sistema de Vigilância Alimentar e Nutricional - SISVAN na assistência à saúde. Série B. Textos Básicos de Saúde. Brasilia - DF, 2008. Available at: http://nutricao.saude.gov.br/docs/geral/protocolo_sisvan.pdf [ Links ]
12. NRC (National Research Council). - Dietary Reference Intake: for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington, D.C., National Academy Press, 2002/2005. [ Links ]
13. ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN 2002; 26 (1 Suppl.): 1SA-138SA. [ Links ]
14. Hulst JM, van Goudoever JB, Zimmermann LJ et al. Adequate feeding and the usefulness of the respiratory quotient in critically ill children. Nutrition 2005; 21 (2): 192-8. [ Links ]
15. O'Leary-Kelley CM, Puntillo KA, Barr J, Stotts N, Douglas MK. Nutritional adequacy in patients receiving mechanical ventilation who are fed enterally. Am J Crit Care 2005; 14 (3):222-31. [ Links ]
16. Oliveira AF, Oliveira FLC, Juliano Y et al. Evolução nutricional de crianças hospitalizadas e sob acompanhamento nutricional. Rev Nutr 2005; 18 (3): 341-348. [ Links ]
17. Brasil. Ministério da Saúde. Coordenação Geral de Alimentação e Nutrição (CGAN). Disponível em: http://nutricao.saude.gov.br/sisvan.php?conteudo=perguntas_respostas_omc#pergunta9. Acessado em maio 20. [ Links ]
18. Araújo LL, Barbosa JM, Ribeiro APG, Santos ACO, Pedrosa F. Nutritional status, dietary intake and serum levels of vitamin C upon diagnosis of cancer in children and adolescents. Nutr Hosp 2012; 27 (2): 496-503. [ Links ]
19. Zalina AZ, Suzana S, A Rahman A Jamal, Noor Aini MY. Nutritional Status of Children with Leukemia Assessing the Nutritional Status of Children with Leukemia from Hospitals in Kuala Lumpur. Mal J Nutr 2009; 15 (1): 45A. [ Links ]
20. Sarni ROS, Carvalho MFCC, Monte CMG, Albuquerque ZP, Souza FIS. Anthropometric evaluation, risk factors for malnutrition, and nutritional therapy for children in teaching hospitals in Brazil. J Pediatr 2009; 85 (3): 223-228. [ Links ]
21. Israel T, Chawanangwa C, Caron HN et al. Nutrition status at admission of children with cancer in Malawi. Pediatric Blood Cancer 2008; 626-628. [ Links ]
22. Ness KK, Gurney JG. Adverse late effects of childhood cancer and its treatment on health and performance. Annu Rev Public Health 2007; 28: 279-302. [ Links ]
23. Monteiro IMU. Tratamento da leucemia linfóide aguda em meninas: repercussões sobre o desenvolvimento puberal e o crescimento. [Mestrado]. São Paulo. Unicamp, 1994. [ Links ]
24. Andrassy RJ, Chwals WJ. Nutritional support of the pediatric oncology patient. Nutrition. 1998; 14 (1): 124-9. [ Links ]
25. Bowman LC, Williams R, Sanders M, Ringwald-Smith K, Baker D, Gajjar A. Algorithm for nutritional support: experience of the metabolic and infusion support service of St. Jude Children's Research Hospital. Int J Cancer 1998; 11: 76-80. [ Links ]
26. Bankhead R, Boullata J, Brantley S et al. ASPEN. Enteral Nutrition Practice Recommendations. JPEN 2009; 33; 122. [ Links ]
27. Pietsch JB, Connie F. Children with Cancer: Measurements of Nutritional Status at Diagnosis. Nutr Clin Pract 2000; 15 (4): 185-188. [ Links ]
28. González JCM, Montiel BE. Complicaciones gastrointestinales en el paciente crítico. Nutr Hosp 2007; 22 (Suppl. 2): 56-62. [ Links ]
29. Nind G, Chen W-H, Protheroe R et al. Mechanisms of gastroesophageal reflux in critically ill mechanically ventilated patients. Gastroenterology 2005; 128: 600-6. [ Links ]
30. Ezge FS, Tumer L, Cinasal G et al. Effect of enteral and parenteral nutrition on growth parameters of hospitalized Turkish children. Indian Pediatr 2005; 42 (17): 469-472. [ Links ]
31. Bozzetti F. Is enteral nutrition a primary therapy in cancer patients? Gut 1994; (Suppl. 1): S65-S68. [ Links ]
32. Garófolo A. Diretrizes para terapia nutricional em crianças com câncer em situação crítica. Rev Nutr 2005; 18 (4): 513-527. [ Links ]
33. Peter JV, Moran JL, Philips-Hughes J. A meta analysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med 2005; 33(1): 213-20. [ Links ]
34. Vila BG, Grau T. La nutrición enteral precoz en el enfermo grave. Nutr. Hosp 2005; 20 (2): 93-100. [ Links ]
![]() Correspondence:
Correspondence:
Janine Maciel Barbosa.
Departamento de Nutrição.
Rua dos Coelhos, 300.
Boa Vista, CEP 50070-550. Recife, PE, Brasil.
E-mail: janinebarbosa@gmail.com
Recibido: 24-III-2012.
Aceptado: 27-III-2012.