Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.27 no.5 Madrid sep./oct. 2012
https://dx.doi.org/10.3305/nh.2012.27.5.6053
Effect of a hypocaloric diet with a commertial formula in weight loss and quality of life in obese patients with chronic osteoarthritis
Efecto de una dieta hipocalórica con una fórmula comercial en la pérdida de peso y calidad de vida en pacientes con osteoartritis crónica
D. A de Luis1, O. Izaola1, M García Alonso2, R. Aller1, G. Cabezas1 and B. de la Fuente1
1Center of Investigation of Endocrinology and Clinical Nutrition, Medicine School. Unit of Investigation
2Dep. Traumatology. Hospital Rio Hortega. University of Valladolid. Valladolid. Spain
ABSTRACT
Background: The aim of our study was to evaluate in patients with obesity and chronic osteoarthritis the impact on quality of life and metabolic control of a dietary intervention with a hypocaloric commercial formula.
Methods: A sample of 55 obese patients with chronic osteoarthritis was enrolled. The study consisted of a 12-week weight reduction program where the participants received an oral diet replaced with 2 bricks of Optisource Plus®.
Results: In order to assess the effect of weight loss on different parameters, patients were divided in two groups by the median of weight loss percentage (9%); group 1 (< 9%) and group 2 (> 9%). In group 2, patients showed an improvement in total SF-36 score (4.0 ± 6.1 points), physical function domain of SF 36 (1.8 ± 3.4 points), role physical domain of SF 36 (0.6 ± 1.6 points) and vitality domain of SF 36 (2.7 ± 4.6 points) improved. Total score of WOMAC test (- 8.2 ± 15.0 points), function domain of WOMAC test (- 6.5 ± 10.6 points) and stiffness domain of WOMAC test (-0.7 ± 2.1 points) improved, too.
Conclusion: The effect on metabolic response, functionality and quality of life was better in patients with a percentage of weight loss > 9% than patients with a lower weight loss.
Key words: Cardiovascular risk factors. Hypocaloric diet. Obesity. Osteoarthritis. Quality of life.
RESUMEN
Introducción: El objetivo de nuestro estudio fue evaluar en pacientes obesos con osteoartritis crónica el impacto sobre la calidad de vida y el control metabólico de una intervención dietética con una formula comercial hipocalórica.
Material y métodos: Se evaluó una muestra de 55 pacientes obesos con osteoartritis crónica. EL estudió consistió en un programa de 12 semanas de reducción de peso en el que los paciente recibían al día dos envases de Optisource Plus®.
Resultados: Se dividieron a los pacientes en dos grupos, uno que perdió menos de un 9% (grupo 1) y el grupo 2 (más de 9% de pérdida de peso). Los pacientes en el grupo 2 mostraron una mejoria en la puntuacion total del test de calidad de vida SF-36 (4,0 ± 6,1 puntos), en el campo de la función física del SF 36 (1,8 ± 3,4 puntos), el en campo del componente físico del SF 36 (0,6 ± 1,6 puntos) y en el campo de la vitalidad del SF 36 (2,7 ± 4,6 puntos). También mejoraron de manera significativa, la puntuación total del test específico de calidad de vida para osteoartritis WOMAC (-8,2 ± 15,0 puntos), el dominio funcional del test WOMAC (- 6.5+/-10.6 puntos) y el dominio de la rigidez del test WOMAC (-0,7 ± 2,1 puntos).
Conclusión: El efecto sobre la calidad de vida fue superior en el grupo con porcentaje de pérdida de peso > 9% con la formula hipocalórica comercial.
Palabras clave: Factores de riesgo cardiovascular. Dieta hipocalórica. Osteoartritis. Calidad de vida.
Introduction
Epidemiological studies have shown obesity to be an important risk factor for the development of knee and hip osteoarthritis (OA).1 In fact, obesity is probably the single most important risk factor for the development of severe OA of the knee, more so than other potential predisposing factors, including heredity.2 Obesity will increase the load on the knee and hip, the resulting effect on the joint could well be responsible for the degeneration of the cartilage as measured by grade of severity in a study of OA patients.3
The obesity epidemic is the wide of the century, with a multifactor origin. For example in our country the prevalence of obesity stands at 13%, overweight over 30%4 and in patients with chronic osteoarthritis, obesity is highly prevalent.5 In these patients the surgical replacements of the hip or knee are usually frequent, as a solution to the pain and the inability of walking. Orthopaedic surgery is not risk-free, and there were more frequent blood losses and venous thrombosis in obese patients.6-7
With this increasing prevalence of obesity, it is essential to develop and assess suitable treatment strategies which will result in long-term weight reduction and maintenance of weight loss.8 Weight loss is difficult to achieve. Typically, a weight loss diet would have a deficit of 500 kcal/day below the current requirement for energy balance, leading to a weight reduction of 0.5 kg per week. For example, Larsen et al.9 evaluated 130 patients with a weight loss program before a total hip arthroplasty, this protocol reached a weight reduction for 73% of patients with an average loss of 8.6 kg. Pekkarinen et al.10 evaluated a total of 30 patients with a very low caloric diet (VLCD) for 7-24 weeks in obese patients awaiting orthopaedic surgery. The average weight loss was 19.6 kg, co-morbidities associated with surgery or other cardiovascular risk factors were not assessed.
Clinically, OA causes painful joints and is a leading cause of impaired mobility in the elderly; most patients with symptomatic osteoarthritis have limitations in function that prevent from engaging in their usual activities.11 The evaluation of quality of life in these patients after weight loss is an interesting area of research.
If we take into account the high prevalence of obesity, along with the increasingly used orthopaedic surgery for the treatment of chronic osteoarthritis, as well as the difficulty to perform their usual activities, the use of protocols for weight loss in these patients to improve quality of life is more than justified.
The aim of our study was to evaluate in patients with obesity and chronic osteoarthritis (hip or knee), the impact on quality of life, pain, weight loss and metabolic control of a dietary intervention with a hypocaloric commercial formula (Optisource Plus®).
Material and methods
A sample of 55 obese patients (BMI > 30) with chronic osteoarthritis was enrolled with a non-probability sampling process, starting recruitment in January 2011 and completed follow-up of patients in May 2012. These patients were studied in a Clinical Nutrition Unit, referred by the Department of Traumathology with the diagnosis of chronic osteoarthritis of the knee or hip; all patients signed an informed consent and the protocol was approved by the ethics committee of the Centre.
Individuals with confirmed knee or hip osteoarthritis according to standing radiographs were eligible for inclusion,12 and obesity as defined by a body mass index (BMI) > 30 kg/m2. Exclusion criteria were: a previous history of ischemic cardiovascular disease or stroke in the previous 36 months, raising the cholesterol > 300 mg/dl, triglycerides > 400 mg/dl and the taking of any of the following medications; sulfonylurea, thiazolidinediones, insulin, glucocorticoids, inhibitors of angiotensin converting enzyme, receptor antagonists, angiotensin II or nutritional supplement.
Procedure
The study consisted of a 12-week weight reduction program where the participants received an oral diet replaced with 2 bricks of Optisource Plus® each day (lunch and dinner times) (1,109.3 kcal/day, 166.4 grams of carbohydrates (60 %), 63 g protein (23%), 21.3 g of 17% fat). The composition of Optisource Plus® (1 envelope) is as follows; 211 calories, 17.55 grams protein, 4.5 grams fat (1.25 grams saturated, 1.75 grams monounsaturated, 1.5 grams polyunsaturated) and 25 4 grams of carbohydrates.
Weight, blood pressure, basal glucose, insulin, HOMA, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides blood were measured at baseline and 12 weeks after the treatment. Each participating patient completed the Spanish version of the Short Form-36 test (SF-36), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and a visual analog scale of joint pain (0 = without pain untill 10 = maximum pain) at baseline and 12 weeks after the treatment, too.
Assays
Serum total cholesterol and triglyceride concentrations were determined by enzymatic colorimetric assay (Technicon Instruments, Ltd., New York, N.Y., USA), while HDL cholesterol was determined enzymatically in the supernatant after precipitation of other lipoproteins with dextran sulphate-magnesium. LDL cholesterol was calculated using Friedewald formula.
Plasma glucose levels were determined by using an automated glucose oxidase method (Glucose analyser 2, Beckman Instruments, Fullerton, CA). Insulin was measured by enzymatic colorimetry (Insulin, WAKO Pure-Chemical Industries, Osaka, Japan) and the homeostasis model assessment for insulin sensitivity (HOMA) was calculated using these values.13
Anthropometric measurements
Body weight was measured to an accuracy of 0.1 kg and body mass index computed as body weight/ (height2). Waist (narrowest diameter between xiphoid process and iliac crest) and hip (widest diameter over greater trochanters) circumferences were measure also to derive waist-to hip ratio (WHR). Tetrapolar body electrical bioimpedance was used to determine body composition.14 An electric current of 0.8 mA and 50 kHz was produced by a calibrated signal generator (Biodynamics Model 310e, Seattle, WA, USA) and applied to the skin using adhesive electrodes placed on right-side limbs. Resistance and reactance were used to calculate total body water, fat and fat-free mass.
Dietary intake and habits
The subjects' nutritional intake was assessed prospectively by analysis of written food records. All subjects enrolled in the study were instructed to record their daily dietary intake for three days, including a weekend day. Handling of the dietary data was by means of a personal computer equipped with personal software incorporating use of food scales and models to enhance portion size accuracy. Records were reviewed by a dietitian and analysed with a computer-based data evaluation system. National composition food tables were used as references.15 Regular aerobic physical activity (walking was allowed, no other exercises) was maintained during the period study (120-180 minutes at least 60% of maximal heart frequency).
Quality of life and joint pain
The Short Form test (SF-36) is a patient-reported questionnaire that assesses health related quality of life in eight domains: physical function, role physical, bodily pain, general health, vitality, social function, role emotional, and mental health. The range of possible scores is 0 to 100, with 100 representing the best possible score. The SF-36 has been widely validates as a generic health-related quality-of-life outcome measure.
The WOMAC is a patient-reported outcome measure designed to determine function related to arthritic disorders involving the knee and hip. It consists of forty-one items divided into three domains: pain, stiffness, and physical function. The minimum score is 0 and the maximum is 96, with lower scores indicating better function. This measure has been validated and shown to be responsive and reliable among adults for the treatment of osteoarthritis.16
Joint pain was assessed with a visual analog scale, the punctuation ranged from (0 no pain) to 10 (maximum pain).
Statistical analysis
Sample size was calculated to detect differences over 5 points in quality of life score of WOMAC test with 90% power and 5% significance (n = 55). The results were expressed as average ± standard deviation. The distribution of variables was analyzed with Kolmogorov-Smirnov test. Quantitative variables with normal distribution were analyzed with a two-tailed, paired Student's-t test. Non-parametric variables were analyzed with the W-Wilcoxon test. Qualitative variables were analyzed with the chi-square test, with Yates correction as necessary, and Fisher's test. In order to assess the effect of weight loss on quality of life, joint pain and metabolic parameters, patients were divided in two groups by the median percentage of weight loss (9%). A p-value under 0.05 was considered statistically significant.
Results
A total of 55 patients were selected and completed the entire study (13 males and 42 females). The mean age was 59.5 ± 13,1 years and a body mass index (BMI) average of 38.6 ± 5.7 kg/m2. A hip osteoarthritis was diagnosed in 11 patients and in 44 patients were diagnosed a knee osteoarthritis.
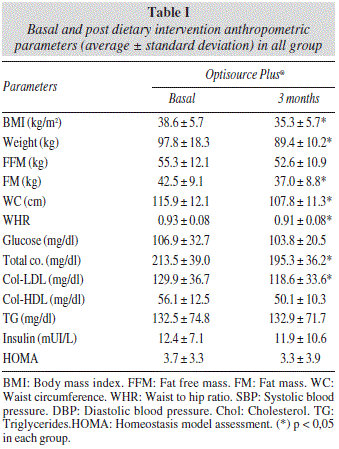
The 55 subjects treated with Optisource plus® basal assessment of nutritional intake with a 3 days written food record showed a calorie intake of 1,685.8 ± 576.1 kcal/day, a carbohydrate intake of 178.3 ± 63.5 g/day, a fat intake of 68.3 ± 27.1 g/day, protein intake of 81.2 ± 26.3 g/day, cholesterol intake of 319.7 ± 147.1 mg/day and fiber intake of 15.9 ± 7.1 g/day. During the intervention, these patients reached the next dietary intakes; calorie intake of 1,077.2 ± 190.2 kcal/day, a carbohydrate intake of 151.1 ± 28.3 g/day, a fat intake of 33.8 ± 10.2g/day, protein intake of 65.1 ± 12.3 g/day, cholesterol intake of 61.8 ± 87.1 mg/day and fiber intake of 16.1 ± 4.3 g/day. Statistical differences were observed between basal and postreatment dietary intakes. Only fiber intake remained unchanged.
Table I shows the differences in anthropometric and biochemical variables. BMI (-3.1 ± 1.5 kg/m2), weight (-7.3 ± 3.8 kg), fat mass (-5.0 ± 3.3 kg), waist circumference (-7.2 ± 4.4 cm) and waist to hip ratio (-0.01 ± 0.04 cm) decreased. Total cholesterol (-13.7 ± 20.7 mg/dl) and LDL-cholesterol levels decreased (-7.3 ± 13.3mg/dl), too.
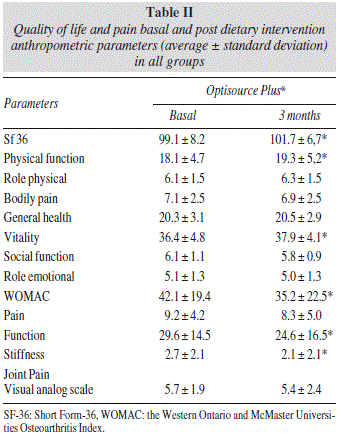
Table II shows the differences in SF-36 questionnaire, WOMAC test and analog visual scale of pain. Total SF-36 score (2.6 ± 6.0 points), physical function domain of SF 36 (1.2 ± 3.2 points), role physical domain of SF 36 (0.26 ± 1.5 points) and vitality domain of SF 36 (1.4 ± 7.0 points) improved. Total score of WOMAC test (- 6.9 ± 15.1 points), function domain of WOMAC test (-5.0 ± 10.2 points) and stiffness domain of WOMAC test (-0.6 ± 2.0 points) improved, too.
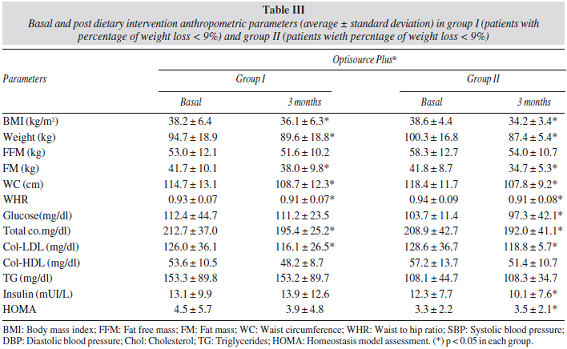
In order to assess the effect of weight loss on quality of life, joint pain and metabolic parameters, patients were divided in two groups by the median percentage of weight loss (9%); group 1 (percentage of weight loss < 9%) and group 2 (percentage of weight loss > 9%). The epidemiological characteristics of group 1 (6 males, 21 females; with an average age of 59.8 ± 11.2 years) was similar than group 2 (7 males, 21 females; with an average age of 59.5 ± 14.1 years). Table III shows the differences in anthropometric variables in group 1 and 2. In group 1, patients showed an improvement of BMI (2.1 ± 0.8 kg/m2), weight (-5.1 ± 2.1 kg), fat mass (-3.7 ± 3.4 kg), waist circumference (-6.1 ± 3.1 cm) and waist to hip ratio (-0.01 ± 0.02 cm) . In group 2, patients showed an improvement of BMI (-4.4 ± 1.4 kg/m2), weight (12.8 ± 1.8 kg), fat mass (-7.2 ± 4.7 kg), waist circumference (-10.6 ± 4.3 cm) and waist to hip ratio (-0.02 ± 0.04 cm). All changes in group 2 were higher than group 1.
Table 3 shows the on differences biochemical parameters. In group 1, patients showed an improvement of total cholesterol (-16.3 ± 21.1 mg/dl) and LDL-cholesterol levels (-9.6 ± 20.5 mg/dl). The improvement of total cholesterol (-17.4 ± 25.5 mg/dl) and LDL-cholesterol levels (-9.9 ± 14.3 mg/dl) was higher in group 2 than group 1. In group 2, insulin (-2.3 ± 8.8 UI/L) and HOMA (-0.8 ± 2.6 UI/L) decreased, too.
Table IV shows the differences in SF-36 questionnaire, WOMAC test and pain analog visual scale in group 1 and 2. In group 1, patients did not reach statistical differences in these three tests. In group 2, patients showed an improvement in total SF-36 score (4.0 ± 6.1 points), physical function domain of SF 36 (1.8 ± 3.4 points), role physical domain of SF 36 (0.6 ± 1.6 points) and vitality domain of SF 36 (2.7 ± 4.6 points) improved. Total score of WOMAC test (-8.2 ± 15.0 points), function domain of WOMAC test (- 6.5 ± 10.6 points) and stiffness domain of WOMAC test (-0.7 ± 2.1 points) improved, too.
Discussion
Our work has been shown that the hypocaloric diet with a commertial formula are able to produce an improvement in weight, body fat, waist circumference, total cholesterol and LDL-cholesterol. The hypocaloric diet with a commertial formula diet resulted in an additional improvement of general quality of life (SF-36 test) and disease specific quality of life (WOMAC-test), without an evident effect on joint pain. However, the better improvement in weight, fat mass, total cholesterol, LDL cholesterol, insulin levels, insulin resistance and quality of life was observed in the group of patients with a percentage of weight loss over 9%.
Previously in the literature have been described good metabolic responses, secondary to hypocaloric diets with commertial formulas.17 An outcome of our work is the improvement of the lipid profile with weight loss, confirming the results in the literature.18 One of the hypotheses that are being proposed to explain this improvement is the change in body composition after weight loss. Low-calorie diets cause a loss of visceral fat, an important way of improving the lipid profile and the different components of metabolic syndrome.19
In our study, patients with higher percentage of weight loss (> 9%) showed an improvement in insulin resistance, the explanation for this is in fact the largest decrease in fat mass and weight with supplemented diet, this result has already been described in protocols using weight loss supplements like our work.20 The use of dietary supplements for weight loss with hypocaloric and very low hypocaloric diets has been very widely in the literature,21-27 demonstrating their safety with the new formulations28 and being recommended in clinical guidelines.29 The use of protocols for weight loss in obese patients with chronic osteoarthritis should be evaluated as these patients have a high rate of comorbidities and a decrease of quality of life. For example, Christensen et al.30 have demonstrated that a weight reduction of 10% improved function by 28% in patients with knee osteoarthritis with a randomized clinical trial using a low-energy diet. These results have been confirmed in other studies with knee osteoarthritis31 and hip osteoarthritis.32
Various scoring systems have been used to report the results of interventions for the treatment of osteoarthritis. These scoring systems can be broadly divided into joint-specific measures and measures of generic quality of life, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is one of the most employed measures of the first time, and the Short Form-36 (SF-36) is one of the most commonly employed measures of the second type. The results of our dietary study demonstrated that patients with a percentage of weight loss > 9% had a greater degree of responsiveness of WOMAC and SF-36 questionnaires than patients with a significant weight loss < 9%. Our study demonstrates that the WOMAC had a greater degree of responsiveness than the SF-36 in obese patients with a percentage of weight loss > 9%. The comparison of the preoperative and postoperative domain scores of both tests revealed significant differences in two of three domains of WOMAC but only in less than half of the domains of the SF-36. Our results are in agreement with previous studies,33 there was a significant clinical improvement (physical function, role physical, vitality by SF-36) and (function and stiffness by WOMAC). It may be deducted from the follow-up results of our study that a better outcome for function, role physical, vitality and stiffness depends on percentage of weight loss achieved (> 9%). These results are similar of a randomized control trial,33 where the intervention group (> 10% percentage of weight loss) showed an improvement in these parameters and the control group (percentage of weight loss < 5%) did not show these results. The lack of response in pain domain of both questionnaires could be due to the short term of our protocol (12 weeks), previous studies33 had a long term follow up (12 months), and then the joint inflammation process could decrease with the maintained weight loss.
Despite these positive results of our work, we are aware of the potential problems of our design as a high Type II error because of the small sample size as well as secondary issues to the lack of a blind design, with possible biases not detected and corrected. Secondly, a limitation is the lack of a control group. Thirdly, the weight loss was a small amount and a short-term; perhaps a more severe weight loss had shown improvements in pain joints. However, our study is one of the few that analyzes the effect of dietary intervention on the functionality and quality of life of obese patients with osteoarthritis.33 From a clinical perspective, these results suggest that physicians can prescribe diet for their obese patients with osteoarthritis, with a wellknown metabolic effect and an interesting effect on quality of life and functionality.
In conclusion, in obese patients with chronic osteoarthritis, weight, fat mass, total cholesterol, LDL cholesterol and some domains of SF-36 and WOMAC questionnaires improved with a hypocaloric diet with a commertial formula. The effect on metabolic response, functionality and quality of life was better in patients with a percentage of weight loss > 9% than patients with a lower weight loss. The short term of the protocol could explain the lack of pain response after a significant weight loss.
References
1. Blagojevic M , Jinks C, Jeffery A et al. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil 2010; 18: 24-33. [ Links ]
2. Coggon D , Reading I, Croft P, et al. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord 2001; 25: 622-7. [ Links ]
3. Felson D T, Goggins J, Niu J et al. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum 2004; 50: 3904-9. [ Links ]
4. Aranceta-Bartrina J, Serra-Majem L, Foz-Sala M, Moreno-Esteban B; Grupo Colaborativo SEEDO. Prevalence of obesity in Spain. Med Clin (Barc) 2005;125 (12): 460-6. [ Links ]
5. Lievense AM, Bierma-Zeinstra SM. Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatology 2002; 41: 1155-1162. [ Links ]
6. Bowditch MG, Villar RN. Do obese patients bleed more? A prospective study of blood loss t total hip replacement. Ann R Coll Surg Engl 1999; 81: 198-200. [ Links ]
7. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology 2003; 99: 552-560. [ Links ]
8. WHO Technical Report Series. The Burden of Musculoskeletal Conditions at the Start of the New Millenium -Report of a WHO Scientifi c Group. Geneva: WHO, 2003. [ Links ]
9. Larsen VH, Sorensen KH. Weight reduction before hip replacement. Acta Othop Scand 1980; 51: 841-844. [ Links ]
10. Pekkarinen T, Mustajoki P. Use of very low-calorie diet in preoperative weight loss: efficacy and safety. Obes Res 1997; 5:595-602. [ Links ]
11. Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med 2006; 354: 841-848. [ Links ]
12. Altman RD. The classification of osteoarthritis. J Rheumatol Suppl 1995; 43: 42-43. [ Links ]
13. Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher Df. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412-414. [ Links ]
14. Lukaski H, Johson PE. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr 1985; 41 (4): 810-7. [ Links ]
15. Mataix J, Mañas M. Tablas de composición de alimentos españoles. Ed: University of Granada, 2003. [ Links ]
16. Thumboo J, Chew LH, Soh CH. Validation of the Western Ontario and Mcmaster University osteoarthritis index in Asians with osteoarthritis in Singapore. Osteoarthritis Cartilage 2001; 9: 440-6. [ Links ]
17. Parker B, Noakes M, Luscombe N, Clifton P. Effect of a high protein high monounsaturated fat weight loss diet on glycaemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25: 425-430. [ Links ]
18. Fujioka S, Matsuzawa Y, Tokunaga K, Kawamoto T, Kobatake T, Keno Y, Kotani K. Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal fat in premenopausal women with visceral fat obesity. Int J Obes 1991; 15: 853-859. [ Links ]
19. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed S. A randomized trial of a low carbohydrate diet for obesity. New Engl J of Med 2003; 348: 2082-2090. [ Links ]
20. Laaksonen DE, Kainulainen S, Rissanen A, Niskanen L. Relationship between changes in abdominal fat distribution and insulin sensitivity during a very low calorie diet in abdominally obese men and women. Nutr Metab Cardiovasc Dis 2003; 13:349-356. [ Links ]
21. Hong K, Li Z, Wang HJ, Elashoff R, Heber D. Analysis of weight loss outcomes using VLCD in black and white overweight and obese women with and without metabolic syndrome. Int J Obes Relat Metab Disord 2005; 29: 436-442. [ Links ]
22. Anderson JW, Konz EC, Frederich RC, Word CL. Long-term weight loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001; 74: 579-584. [ Links ]
23. Saris WHM. Very low calorie diets and sustained weight loss. Obesity Research 2001; 9: 295S-301S [ Links ]
24. Hormiguera X, Barbany M, Carrillo M, Galan A, Herrero P, Foz Salas M. Modificaciones antropométricas y balance de nitrógeno en pacientes con obesidad morbida tratados con dieta muy baja en calorías. Med CLin (Barc) 1991; 96: 401-404. [ Links ]
25. Blanch S, Recasens MA, Solá R, Salas Salvador J. Efecto de una dieta altamente hipocalórica sobre el control de la obesidad mórbida a corto y medio plazo. Med Clin (Bar) 1993; 100: 450-453. [ Links ]
26. Capstick F, Brooks BA, Burns C, Zilkens RR, Steinbeck KS, Yue DK. Very low calorie diet: a useful alternative in the treatment of the obese NIDDM patient. Diabetes Res and Clin Pract 1997; 36: 105-111. [ Links ]
27. Lewis MC, Philips ML, Stlavotinek JP, Kow L, Thompson CH, Toouli J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg 2006; 16: 697-701. [ Links ]
28. Sumithran P, Proietto J. Safe year long use of a very low calorie diet for the treatment of severe obesity. Med J Aust 2008; 188:366-368. [ Links ]
29. Jordi Salas-Salvadó, Miguel A. Rubio, Montserrat Barbany, Basilio Moreno y Grupo Colaborativo de la SEEDO. Consenso SEEDO 2007 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica. Med Clin (Barc) 2007; 128 (5): 184-96. [ Links ]
30. Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? Randomized trial. Osteoarthritis Cartilage 2005; 13: 20-27. [ Links ]
31. Messier SP, Loeser RF, Miller GD, Morgan TM, Jack Rejeski W, Sevick MA, Ettinger WH, Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis. Arthritis and Rheumatism 2004; 50: 1501-1510. [ Links ]
32. Paans N, van den Akker-Scheek I, van der Meer K, Bulstra S, Stevens M. The effects of excercise and weight loss in overweight patients with hip osteoarthritis: design of a prospective cohort study. BMC Musculoskeletal Disorders 2009; 10:24-35. [ Links ]
33. Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomized controlled trial. Ann RheumDis 2011; 70: 1789-1803. [ Links ]
![]() Correspondence:
Correspondence:
D. A. de Luis
Professor of Endocrinology and Nutrition
Director of Center of Investigation of Endocrinology and Clinical Nutrition
Medicine School. University of Valladolid
C/Los perales, 16
47130. Simancas. Valladolid. Spain
E-mail: dadluis@yahoo.es
Recibido: 3-V-2012
1.a Revisión: 13-VII-2012
Aceptado: 24-VII-2012