Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.1 Madrid ene./feb. 2013
https://dx.doi.org/10.3305/nh.2013.28.1.6258
ORIGINAL
Zinc in plasma and breast milk in adolescents and adults in pregnancy and pospartum; a cohort study in Uruguay
Zinc en el plasma y leche materna en embarazadas adolescentes y adultas y en el postparto; estudio de cohorte en Uruguay
Cecilia Seven1,2, Michael Hambidge3, Nancy Krebs3, Rafael Alonso4 and Eduardo Atalah5
1Departamento de Medicina Preventiva y Social, Universidad de la República. Montevideo. Uruguay.
2CLAP/OPS/OMS (A.I.), Hospital de Clínicas. Montevideo. Uruguay.
3Department of Pediatrics, Health Sciences Center, University of Colorado. Denver. USA.
4Departamento de Métodos Cuantitativos, Facultad de Medicina, Universidad de la República. Montevideo. Uruguay.
5Departamento de Nutrición, Facultad de Medicina, Universidad de Chile. Santiago de Chile.
ABSTRACT
Objective: To assess if age is a risk factor for low zinc nutritional status in pregnancy, postpartum and in breast milk concentration, and the association between mother zinc plasma level with zinc milk concentration.
Design: Cohort study comparing adolescents with adult women, with < 14 weeks of gestation at first prenatal care. Socio demographic and plasma zinc data were collected at that moment and at postpartum time (4 + 1 month). Milk zinc concentrations were also measured at 4th month postpartum.
Setting: Women were recruited from 16 public primary health care services in Uruguay
Subjects: 151 adolescents and 161 adult women
Results: Adolescent average plasma zinc at < 14 weeks of gestation was 84.4 ± 3.6 μg /dl and did not differ significantly from that for adult women (85.2 ± 13.6 ug/dl). Prevalence of hypozincemia was relatively low with but with no difference by age (14.6% in adolescents and 12.3% in adults).
Zinc concentrations in breast milk were similar for adolescents, 1.24 mg. /L (CI 1.06 to 1.44) and adult women, 1.27 mg./L (CI .1.0-1.46). There was no correlation between plasma zinc and breast milk zinc concentrations in adults and a weak correlation in adolescents (-0.27, p <0.05).
Conclusions: Prevalence of hypozincemia in pregnancy was relatively low but similar in adolescents and adult women. Neither pregnancy nor age had negative consequences over postpartum plasma zinc, nor over breast milk zinc concentrations. No correlation was found between mother s plasma zinc and breast milk levels.
Key words: Pregnancy. Zinc. Adolescent. Breast milk. Uruguay.
RESUMEN
Objetivo: Evaluar la edad como factor de riego para el déficit nutricional de zinc en el embarazo y en el posparto y la correlación entre la concentración de zinc plasmático y de la leche materna.
Diseño: Estudio de cohorte de 151 embarazadas adolescentes y 161 adultas con < 14 semanas de gestación al primer control prenatal, seleccionadas en 16 centros públicos de salud de primer nivel de atención de Uruguay. Se obtuvieron datos socio demográficos y se determinó zinc plasmático al primer control prenatal y 4 meses posparto (± 1 mes). En el último control se midió también la concentración de zinc en la leche materna.
Resultados: La media de concentración de zinc plasmático a las 14 semanas de gestación fue 84.4 ± 3.6 ug. /dl sin diferencias significativas con las adultas (85.2 ± 13.6 ug/dl). La prevalencia de hipozincemia fue relativamente baja, sin diferencias entre los grupos (14.6% en adolescentes y 12.3% en adultas). La concentración de zinc en la leche materna fue similar en adolescentes y adultas (1.2 CI 1.1-1.4 mg. /L en el grupo total). No se encontró correlación entre el nivel plasmático de zinc materno y la concentración en la leche en adultas y una débil correlación en el grupo de adolescentes (-0.27, p <0.05).
Conclusión: La prevalencia de hipozincemia en el embarazo es relativamente baja y similar entre adultas y adolescentes. No se observó relación entre la edad materna y los niveles plasmáticos de zinc post parto en la madre ni en la leche materna. La concentración de zinc plasmático materno no se correlacionó con el zinc en la leche materna.
Palabras clave: Gestación. Zinc. Adolescencia. Leche materna. Uruguay.
Introduction
During adolescence, the last stage of life with accelerated linear growth, nutritional requirements during pregnancy are exceptionally high. There is limited knowledge about changes in plasma zinc generated by additional demand in pregnancy and its relation with maternal age, but some studies showed that plasma zinc levels from teenagers were not significantly different than those from adults1-3.
Different studies show association between maternal zinc deficiency during pregnancy and spontaneous abortions, malformations, low birth weight, intrauterine growth retardation, birth complications, and fetal development4-8.
Zinc concentrations in milk have been shown to be independent of maternal Zn intake and nutritional status, especially in well nourished women9-10, also in women with marginal intakes11-12.
It is still unknown the effects of adolescence on milk Zn concentrations and on Zn status a period of time with high demands for Zn for growth and with frequently marginal quality of diets. The aim of this study was to examine if age is associated with zinc status at early pregnancy and postpartum in plasma and breast milk.
Methods and procedures
We conducted a cohort study in Uruguay comparing 151 adolescents (13-19 years old) with 161 adult women (24-35 years old), with < 14 weeks of gestation at first prenatal control.
Sample size was calculated based in previous studies which showed 98.2 μg/dl of plasma zinc concentration in adults and 94 μg/dl in adolescents, assuming a normal distribution within each group, standard deviation of 11 and a difference between means of 4. Considering a power of 0.8 and type I error probability of 0.05 we need to study 120 subjects in each group to be able to reject the null hypothesis 13-14.
Women were recruited from sixteen public primary health centers and invited to participate when they attended for their first prenatal visit. The inclusion criteria were age, 10-19 years old for adolescents and 24-35 for adults; single pregnancy; without pathologies; < 14 weeks gestational age at first prenatal control measured by last date of menstruation if known or gestational age estimated by ultrasound if unknown; agreement to participate and informed consent signed. We included all mothers who met these criteria regardless their ethnicity, socio-economic and cultural level, marital status, parity, tobacco or alcohol consumption. Exclusion criteria were twin pregnancies and mothers with previously diagnosed of chronic diseases. The study was submitted to local and Pan-American Health Organization (PAHO) ethical approval, informed consent were obtained and signed, and in all cases confidentiality had been ensured.
Data were obtained at < 14 weeks gestation age, and 4 ± 1 months postpartum. At the first prenatal visit demographic data were obtained including age, race, marital status, and schooling. At 4 ± 1 month postpartum second blood samples were taken at health centers. Patients who were not found at health centers were visited at home to collect data, in many cases mothers were moved to capital city to obtain blood sample. A sample of breast milk also was taken in mothers who were lactating at 4th month. A precise logistic plan was undertaken and monitoring visits were made to assess quality of data collected.
Blood sample
A 7 ml. blood sample was collected by peripheral venipuncture using disposable plastic syringes and stainless steel needles or butterflies if veins were very thin. The samples were collected at morning and at fasting. Samples were transferred to plastic Eppendorf tubes containing heparin. After centrifuging for 10 minutes at 1200-1500 xg, plasma was separated in and transferred to four plastic Eppendorf (0,5 to 0,7 ml/tube) and immediately frozen at -20 oC. The syringes, heparin, and tubes were free of detectable zinc.
Breast milk sample
After washing breast de-ionized water, milk samples of 5 ml. were collected at 5 minutes of feeding by manual expression directly into zinc - free polypropylene containers, and immediately frozen at -20oC until thawed for analysis.
Laboratory assays
All sample analyses were performed at the University of Colorado, Denver. Instructions of United Nations for packing and regulations for shipment were followed. Samples ashing analytical procedures were identical for blood and milk and were analyzed by flame atomic absorption spectrophotometer with a modified Perldn-Elmer 503 fitted with deuterium arc background correction and AS-3 auto sampling system (Perkin Elmer Corp, Norwalk, CT).
The cutoff point used as a reference of low plasma zinc was the proposed by the International Zinc Nutrition Consultative Group (IZiNCG): < 70 μg/dl, which is the lower limit of normality 15.
Statistical analysis
For testing normality, Kolmogorov-Smirnov Z test was used. For comparing quantitative variables between adolescents and adults t-test for independent samples was assessed, and if normality not met, Mann Whitney U test was applied. Summary measures were used as means and standard deviations for normal continuous variables and proportions for categorical ones, analyzing the differences between adolescent and adult women in early pregnancy and postpartum.
Mixed models were used for comparison of zinc values between groups (adolescents and adults) and between stages (first pregnancy control with postpartum). For normalizing and comparing breast milk zinc between groups we calculated the Logarithm (breast milk). For comparing categorical variables, Chi square test was assessed. If expected values were less than 5, categories were joined. For comparing qualitative variables between pregnancy and postpartum, Mc Nemar test was applied. Spearman coefficient was calculated for assessing the correlation between breast milk and plasma zinc. P-values <0.05 was considered significant for all tests. For the statistical analysis it was used SPSS version 15.
Results
312 mothers were recruited and 245 finished inside the study. The causes of lost were: abortion: 34; still birth: 1; deserted: 6 and lost in follow-up: 26. This flowchart shows the follow-up:

Both groups had similar characteristics socio-demographic, years of study, stable union, Caucasian more than 70% and with no difference in gestational age at delivery (table I).
Adolescent mean plasma zinc at <14 weeks of gestation was 84.4 ± 13.6 μg/dl and did not differ from that of adult women (85.2 ± 13.6 μg/dl). Plasma zinc levels were similar when compared recruited mothers with actually followed up with mothers lost in follow-up (p= NS). The prevalence of hypozincemia was relatively low in both groups. When postpartum results were compared with pregnancy results, differences were not found either in prevalence of hypozincemia or of mean plasma zinc (table II).
The majority of women with hypozincemia at < 14 weeks gestation had values within the normal range post-partum and very few normal values at < 14weeks gestation had hypozincemia post-partum, without differences in relation to age (table III).
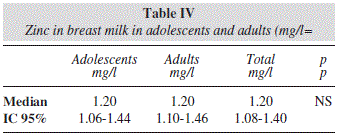
Milk zinc concentrations had similar values in both groups (p= NS) and with the same median value (1.20 ml/L, table IV), and the same declining from 3rd to 5th lactation month (data not shown). When Spearmann coefficient was applied between plasma zinc and milk zinc levels, correlation was not found in adult group (-0.02, NS), and a very weak correlation in adolescent (0.08, p< 0.05). Also no significant difference was found between mother's BMI and milk zinc levels for two groups (data not shown).
Discussion
This is the first study in Uruguay of plasma and milk zinc concentrations in adolescent and adult women. In this study we found a plasma zinc media of 84 ug./dl, which is lower than studies done in other countries as Brazil and Spain but slightly higher of those found in Venezuela and Mexico13-20. We found a prevalence between 7 to 14 % of women with hypozincemia at early pregnancy, defined as values below 70 μg/dl. This prevalence is lower than that found by Meertens in Venezuela, Villapando in Mexico and Sanchez in South Spain but similar as found in Argentine15-16,19. These differences could be due to different dietary habits. In Uruguay meat eating is relatively high, according to Food Balance Sheets of FAO, media zinc consumption for the whole population from this source is 6.46 mg./day21. Sample size could be another explanation, because some of other studies were done over smaller samples than this one, so variances may be wider. Another explanation could be the different methods used to measure zinc in plasma.
Plasma zinc levels did not differ between adolescents and adults in early pregnancy and also in postpartum, so it seems to be that both groups face pregnancy with same nutritional conditions and responded in the same way to the zinc increased demands caused by pregnancy and lactation. Our findings are consistent with the study of Lima de Moraes, who studied 40 adolescents and 40 adults in Brazil, Ruiz who studied 108 pregnant women in Venezuela and Martin Lagos in Spain, who found no association between serum zinc and age in any of three trimesters of pregnancy21-23. Also Neggers found no significant difference related to maternal age and his multiple regression analysis indicates that race, parity, and pregnancy weight were significantly associated with plasma zinc levels adjusted for gestational age24.
In our study we found no significant differences when compared plasma zinc concentration between postpartum and early pregnancy, both groups had the same performance. Also, most of women which began pregnancy with hypozincemia raised their plasma values resulting at 4th month postpartum over 70 μg/dl. A possible reason lies on what Christine Hotz explains in her paper about cutoffs of serum zinc concentrations for assessing zinc status25. This paper was a reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980), and studied variations in zinc concentration by different characteristics, including pregnancy. It described a decline of plasma zinc concentration since very early in pregnancy and suggested a cutoff of 56 μg/dl at first trimester of pregnancy25, 26.
In our study, milk zinc was not correlated to plasma zinc concentration and also mother's nutritional status. Although in the group of adolescent mothers was statistically significant, the correlation found is considered very weak. These findings are similar with some previous studies which found that milk zinc is not affected by plasma values27-29.
Hannan and Rashed showed no significant correlation between zinc intake and mineral concentration in breast milk30, 31. Also other recent studies, one on a sample of malnourished women in Honduras and other on a sample of well-nourished women in Sweden, assessed correlation between concentrations of iron, zinc and copper between plasma and milk. These studies showed no association between mother's plasma zinc and milk zinc measured at 9 months postpartum, which is consistent with our results although we found a weak association en adolescent group32.
A plausible reason appears to be that the drop in plasma zinc during pregnancy may be due to hormonal effects and hemo-dilution. Also this drop in plasma zinc causes increased absorption and some authors showed that depending on quantity of zinc consumed, is the quantity of zinc absorbed 33, 34.
Our study also found a mild declining of milk zinc from 3rd to 5th month of lactation (adolescents and adults) as published in previous studies. As milk zinc concentration changes along post-partum time, it is important to highlight that the spread of sample around 4 month time point was similar between adults and adolescents 35, 36.
Research on nutritional zinc status is still a controversial issue. A review conducted in 2007 suggests that zinc research particularly in pregnant adolescents is still very limited and therefore results are not conclusive37. Another review in 2000, by Janet C King does not show age as a risk factor in the nutritional status of zinc in pregnancy38. The mean age of the adolescents was relatively high and well past growth spurt. It might have been different if the young women had been in stage Tanner 3 or so. In our study the mean of adolescent age was 17 years old.
According to these results, adolescents begin their pregnancy under similar zinc condition of adult ones. In this nutrient body seems to be ready and do not put them in a greater vulnerable position to face pregnancy demands.
As findings in literature show that health outcomes in adolescent pregnant and perinatal outcomes are worse than adults, we must search for other constraints than zinc39.
This study also do not allow to go in depth in the identification of social factors which may be influencing, because both groups are very similar in social conditions, and the design was made to prove other hypothesis. From this perspective we must review clinical practice in nutrition and social policies to address pregnant adolescents and to identify areas where progress is required to increase knowledge in this subject. Our results of similar Body Mass Index between adolescent and adult women support the same conclusion than zinc results, that age by herself appears not to be a risk factor in pregnancy and postpartum40.
One weakness is that inside the sample of adolescent studied were very few under 15 years old, although we carried out an stratified analysis under and over 16 having the same findings (100 women but only 15 cases under 15). It will be important to carry out a study with a sample of women under 15 to see if findings being equal.
We can conclude from this study that neither pregnancy nor age over 16 had negative consequences over postpartum plasma zinc, nor over breast milk zinc concentrations.
References
1. Lima de Moraes M, de Faria Barbosa R, Santo R, et al. Maternal-Fetal Distribution of Calcium, Iron, Copper and Zinc in Pregnant Teenagers and Adults. 2010 Biological Trace Element Research.; DOI: 10.1007/s12011-010-8649-6. [ Links ]
2. Maia PA, Figueredo RC, Anastasio AS, et al. Iron, zinc, folate and vitamin B12 nutritional status and milk composition of low-income Brazilian mothers. Eur J Clin Nutr 1989; 43: 253-66. [ Links ]
3. Ortega RM, Andrés P, Martínez RM, et al. Zinc levels in maternal milk: the influence of nutritional status with respect to zinc during the third trimestre of pregnancy. Eur J Clin Nutr 1997; 51: 253-8. [ Links ]
4. Hotz C, Brown KH. International Zinc Nutrition Consultative Group (IZINCG). Technical document No 1 Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004; 25: S99-199 2. [ Links ]
5. Danesh A, Janghorbani M, Mohammadi B. Effects of zinc supplementation during pregnancy on pregnancy outcome in women with history of preterm delivey: a double - blind randomized, placebo-controlled trial. J Matern Fetal Neonatal Med 2010; 23: 403-8. [ Links ]
6. Hess SY, King JC. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr Bull 2009; 30: S60-78. [ Links ]
7. Azman MS, Wan Saudi WS, Ilhami M, Mutalib MS. Zinc intake during pregnancy increases the proliferation at ventricular zone of newborn brain. Nutr Neurosc 2009; 12: 9-12. [ Links ]
8. Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev 2007; 18: CD000230. [ Links ]
9. Krebs NF, Hambidge KM, Jacobs MA, et al. The effects of a dietary zinc supplement during lactation on longitudinal changes in maternal zinc status and milk zinc concentrations. Am J Clin Nutr 1995; 41: 560-570. [ Links ]
10. Krebs NF, Reidinger CJ, Hartley S, et al. Zinc supplementation during lactation: Effects on maternal status and milk zinc concentrations. Am J Clin Nutr 1995; 61: 1030-6. [ Links ]
11. Sian L, Krebs NF, Westcott JE, et al. Zinc homeostasis during lactation in a population with low zinc intake. Am J Clin Nutr 2002; 75: 99-103. [ Links ]
12. Dhonukshe-Rutten RA, Vossenaar M, West CE, Schürman K, Solomons NW. Day to Day variations of Iron, Zinc and Cooper in Breast Milk of Guatemalan Mothers. J Ped Gastroenterol Nutr 2005; 40: 128-134. [ Links ]
13. Saliba LF, Tramonte VL, Faccin M, Gersol L. Zinc no plasma e eritrocito de atletas profissionais de urna equipe feminina brasileira de voleibol. Nutr 2006; 19: 581-590. [ Links ]
14. International Zinc Nutrition Consultative Group (IZINCG). Assessment of the risk of zinc deficiency in populations and options for its control. Hotz C, Brown KH, eds. Food Nutr Bull 2004. 25:S91: S204. [ Links ]
15. Meertens L, Solano L, Sánchez A. Hemoglobina, ferritina y zinc sérico de mujeres en edad reproductiva. Su asociación con el uso de anticonceptivos. An Venez Nutr 2002; 15: 5-10. [ Links ]
16. Villalpando S, García Guerra A , Ramírez Silva, et al. Estado de hierro, zinc y yodo en niños menores de 12 años y en mujeres de 12-49 años de edad en México: una encuesta probabilística nacional. Salud Pública Méx 2003; 45: 520-529. [ Links ]
17. De Mateo Silleras B, Pérez García A, Miján de la Torre A. The zinc status in a selected Spanish population. A multivariate analysis. Nutr Hosp 2000; 15: 32-41. [ Links ]
18. Diaz Romero C, Henríquez Sánchez P, López Blanco F, et al. Serum copper and zinc concentrations in a representative sample of the Canarian population. J Trace Elem Med Biol 2002; 16: 75-81. [ Links ]
19. Sánchez C, Lopez-Jurado M, Planells E, Llopis J, t al. Assessment of iron and zinc intake and related biochemical parameters in an adult Mediterranean population from southern Spain: influence of lifestyle factors. J Nutr Biochem 2009; 20: 125-31. [ Links ]
20. Weisstaub AR, Meméndez AM, Montemerlo H, et al. Zinc plasmático, cobre sérico y zinc y cobre eritrocitarios en adultos sanos de Buenos Aires. Acta Bioquím Clin Latinoam 2008; 42:315-23. [ Links ]
21. Food and Agriculture Organization of United Nations 2010 http://faostat.fao.org, being consulted at November 13th, 2010. [ Links ]
22. Ruiz N, Meertens L, Peña E, Sánchez A, Solano, L. Comportamiento de los niveles séricos de zinc durante el embarazo. Arch Latinoam Nutr 2005; 55: 235-244. [ Links ]
23. Martin-Lagos F, Navarro-Alarcón M, Terres-Martos C. Zinc and copper concentations in serum from spanish women during pregnancy. Biol Trace Elem Res 1998; 61: 61-70. [ Links ]
24. Neggers Y, Dubard M, Godelberg R, Tamura T, Johnston K, Copper R, et al. Factors influencing plasma zinc levels in low-income pregnants women. Biol Trace Elem Res 1996; 55: 127-135. [ Links ]
25. Hotz C, Peerson JM & Brown KH Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr 2003; 78: 756-764. [ Links ]
26. Rosalind S. Gibson, Sonja Y. Hess. Christine Hotz and Kenneth H. Brown. Indicators of zinc status at the population level: a review of the evidence. British J Nutr 2008; 99: S14-23. [ Links ]
27. Donangelo CM, Trugo NM, Koury JC. Iron, zinc, folate and Vti. B12 nutritional status and milk composition of low-income Brazilian mothers. Eur J Clin Nutr 1989; 43: 253-66. [ Links ]
28. Feeley RM, Eitenmiller RR, Jones JB Jr, et al. Copper, iron and zinc contents of human milk at early stages of lactation. Am J Clin Nutr 1983; 37: 443-8. [ Links ]
29. Krebs N, Hambidge MK. Complementary feeding: clinically relevant factors affecting timing and composition, milk. J Clin Nutr 2007; 85: 639S-45 [ Links ]
30. Hannan MA, Faraji B, Tanguma J, et al. Copper, selenium, and zinc concentrations in human milk during the first three weeks of lactation. Biol Trace Elem Res 2009; 127: 6-15. [ Links ]
31. Rached de Paoli I, Henríquez G., Aguaje A. Niveles séricos de zinc y su relación con la ingesta de nutrientes en gestantes eutróficas. An Venez Nutr 2004; 17: 5-11. [ Links ]
32. Domellof M, Lonnerdal B, Dewey KG, et al. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am J Clin Nutr 2004; 79: 111-115. [ Links ]
33. Donatelo C, Vargas C, Woodhouse LR. Zinc absortion and kinetics during pregnancy and lactation in Brazilian women. Am J Clin Nutr 2005; 82: 118-24. [ Links ]
34. Faceb, Kina JC, Shames DM, et al. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr 2001; 74: 116-24. [ Links ]
35. Abulrazzzq YM, Osman N, Nagelkerke N, et al. Trace element composition of plasma and breast milk of well - nourished women. J Environ Sci Health A Tox Hazard Subsm Environ Eng 2008; 43: 329-34. [ Links ]
36. Al-Awadi FM, Srikumar TS. Trace-element status in milk and plasma of Kuwaitti and non-kuwaiti lactating mothers. Nutrition 2000; 16; 1069-73. [ Links ]
37. Brown KH, Engle-Stone R, Krebs NF, et al. Dietary intervention strategies to enhance zinc nutrition: promotion and support of breastfeeding for infants and young children. Food Nutr Bull 2009; 30: S1444-71. [ Links ]
38. King J. Determinants of maternal zinc status during pregnancy. Am J Clin Nutr 2000; 71: 1334S-1343S. [ Links ]
39. Iacobelli S, Robillard PY, Gouyon JB, Hulsey TC, Barau G, Bonsante F. Obstetric and neonatal outcomes of adolescent primiparous singleton pregnancies: a cohort study in the South of Reunion Island, Indian Ocean. J Matern Fetal Neonatal Med 2012. [ Links ]
40. Severi C, Alonso R, Atalah E. Cambios en el Índice de Masa Corporal entre adolescentes y adultas entre el embarazo y posparto. Arch Latinoam Nutr 2009; 59: 227-34. [ Links ]
![]() Correspondence:
Correspondence:
Eduardo Atalah Samur.
Facultad de Medicina,
Universidad de Chile.
E-mail: eatalah@med.uchile.cl
Recibido: 22-X-2012.
Aceptado: 19-XII-2012.