Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.2 Madrid mar./abr. 2013
https://dx.doi.org/10.3305/nh.2013.28.2.6183
ORIGINAL
Fatty acids intake and immune parameters in the elderly
Ingesta de ácidos grasos y parámetros inmunes en ancianos
Sonia González1, Patricia López1,2, Abelardo Margolles2, Ana Suárez1, Ángeles M. Patterson1, Adriana Cuervo1, Clara G. de los Reyes-Gavilán2 and Miguel Gueimonde2
1Department of Functional Biology. University of Oviedo. Oviedo. Asturias. Spain.
2Department of Microbiology and Biochemistry of Dairy Products. Instituto de Productos Lácteos de Asturias (IPLA-CSIC). Villaviciosa. Asturias. Spain.
This work was funded by Biopolis SL. within the framework of the e-CENIT Project SENIFOOD from the Spanish Ministry of Science and Innovation. We show our greatest gratitude to all the volunteers participating in the study.
ABSTRACT
Introduction: The rapid increase on life-expectancy represents a major challenge and economic burden for modern societies. Several studies have focused on the effects of polyunsaturated fatty acids (PUFA) upon the immune system; however less attention has been paid to the effects of monounsaturated fatty acids (MUFA). In this work we investigated the relationship of habitual consumption of different types of fatty acids with different immune parameters in the elderly.
Subjects and methods: 40 institutionalized elderly (76-95 y) and 35 home-living middle-age subjects (57-65 y) were recruited. Dietary intakes of macronutrients, fiber and fatty acids, as well as immune parameters such as serum cytokines levels (IL-10, TNF-α, IL-8, IL-17, TGF-β and IL-12), phagocytic activity and cytotoxic NK activity, were determined.
Results: Elderly subjects had a lower intake of total lipids. MUFA intake was significantly lower in the elderly group than in middle-age adults whilst the contrary was true for PUFA. MUFA intake in the elderly was found to be positively associated with IL-12 (β = 0.879) and TNF-α (β = 0.789) serum concentrations, whilst PUFA intake was inversely related to levels of IL-12 (β = -0.534). These associations were not observed in the middle-age group.
Conclusion: MUFA intake may contribute to the pro-inflammatory status present in the elderly. It may be advisable to develop future nutrient recommendations specific for elderly taking into account immune parameters.
Key words: Fatty acids. MUFA. Elderly. Immune parameters. Cytokines.
RESUMEN
Introducción: El rápido aumento de la esperanza de vida en las sociedades desarrolladas representa un gran desafío y supone una elevada carga económica. Numerosos trabajos han estudiado los efectos de los ácidos grasos poliinsaturados (AGP) sobre el sistema inmune, sin embargo los efectos de los ácidos grasos monoinsaturados (AGM) han recibido mucha menos atención. En este trabajo se investigó la relación del consumo habitual de los diferentes tipos de ácidos grasos con diversos parámetros inmunológicos en ancianos.
Individuos y métodos: Se reclutaron 40 ancianos institucionalizados (79-95 años) y 35 individuos de mediana edad (57-65 años) que residían en sus hogares. Se determinó la ingesta diaria de macronutrientes, fibra y ácidos grasos, así como diversos parámetros inmunes; niveles séricos de citoquinas (IL-10, TNF-α, IL-8, IL-17, TGF-β, IL-12), actividad fagocítica y actividad citotóxica de células NK.
Resultados: Los voluntarios ancianos presentaron una menor ingesta de lípidos totales. La ingesta de AGM fue significativamente menor en el grupo de ancianos que en los adultos de mediana edad, mientras que lo contrario fue cierto para los AGP. La ingesta de AGM en ancianos se asoció positivamente con las concentraciones de IL-12 (β = 0,879) y TNF-α (β = 0,789), mientras que la ingesta de AGP mostró una relación inversa con los niveles de IL-12 (β = -0,534). Estas asociaciones no fueron observadas en el grupo de mediana edad.
Conclusión: La ingesta de AGM podría contribuir al estado pro-inflamatorio presente en los ancianos. Sería aconsejable desarrollar recomendaciones nutricionales específicas para ancianos teniendo en cuenta parámetros inmunológicos.
Palabras clave: Ácidos grasos. Ácidos grasos monoinsaturados. Ancianos. Parámetros inmunes. Citocinas.
Abbreviations
EPIC: European Prospective Investigation into Cancer and Nutrition. FA: Fatty acids.
FFQ: Food frequency questionnaire.
IL-10: Interleukin 10.
IL-12: Interleukin 12.
IL-17: Interleukin 17.
IL-8: Interleukin 8.
MUFA: Monounsaturated fatty acids.
NK: Natural killer cells.
PBMCs: Peripheral blood mononuclear cells.
PUFA: Polyunsaturated fatty acids.
SFA: Saturated fatty acids.
TGF-β: Transforming growth factor-beta.
TNF-α: Tumor Necrosis Factor-alpha.
Introduction
During the last century there has been a continuous rise on life-expectancy,1 representing a challenge and economic burden for modern societies. Aging has been related with altered immune function, or more precisely age-associated immune deregulation,2 including decreased proliferative response to mitogens, low activity of natural killer (NK) cells3,4 and increased levels of pro-inflammatory cytokines.5,6 These changes on immune function, commonly called immunosenescence, may explain the high susceptibility of elderly people to disease. Correction of these age-related changes constitutes a target for the development of nutritional intervention strategies directed to the elderly population.
Although under-nutrition appears to be one of the main factors that could induce altered immune responses in aged individuals,2 during the last decade several studies have evaluated the effects of amount, type and quality of dietary fat on the human immune response.7 Dietary fatty acids (FA) have been considered as regulators of inflammatory burden, n-6 polyunsaturated fatty acids (PUFA) being reported as having inflammatory capacities, while the n-3 series present anti-inflammatory effects.8,9 However, less attention has been paid to the effects of monounsaturated fatty acids (MUFA) upon the immune system. MUFA have been traditionally considered as neutral fatty acids and have often been used as placebo in studies investigating the anti-inflammatory properties of other FA.10 However, they may also be able to modulate the immune system11 and have been used in the resolution or attenuation of disease.12
The net effect of dietary fat on immune response is likely an outcome of the interaction between several factors, including total fat, type of fat, ratios between dietary FA and nutrient status. The components in foods that improve immune functions are still far from fully understood and, therefore, studies on the effect of diet are still required. We investigated here the effects of consumption of FA on different immune parameters in elderly.
Subjects and methods
Participants
The study sample included 75 volunteers from Asturias region (Northern Spain). The elderly group was comprised by 40 institutionalized volunteers (31 females, 9 males; age from 76 to 95 years, mean 81.8 years). A group of 35 middle-age subjects (25 females, 10 males; 57 to 65 years old, mean 60.5) living at their homes, was included for comparison. Exclusion criteria were previous diagnosis of cancer, autoimmune or digestive diseases and consumption of probiotics/prebiotics or antibiotics during the previous month. Participants were mentally and physically able to participate in the study and gave their written informed consent. Ethical approval was obtained from the Committee on Ethical Research of the Oviedo University Hospital.
Nutritional assessment
Dietary intake was assessed by means of a semiquantitative food frequency questionnaire (FFQ), method that has been widely used by our group in other studies. Trained dieticians asked about cooking practices, number and amount of ingredients used in each recipe, as well as questions concerning menu preparation (e.g., type of oil used, type of milk). During an interview, subjects were asked item-by-item whether they usually ate each food and, if so, how much they used to eat. For this purpose, 3 different serving sizes of each cooked food were presented in pictures to the participants so that they could choose from up to 7 serving sizes (from "less than the small one" to "more than the large one"). For some of the foods consumed, amounts were recorded in household units, by volume, or by measuring with a ruler. Food intake was analyzed for energy and macro-and micronutrients content by using the nutrient Food Composition Tables developed by the CSIC.13 Fatty acids were obtained from the Food Composition Tables of the European Prospective Investigation into Cancer and Nutrition (EPIC) group of Spain. Subjects were also asked about whether they were following a special diet due to health problems such as chewing impairment, diabetes or hypercholesterolemia.
Immune measurements
A heparinized whole blood sample was taken from each subject immediately after the nutritional assessment period. The capacity of blood leucocytes to phagocytose Escherichia coli was quantified in a FACSCanto II Flow Cytometer (Becton Dickinson, BD Biosciences, San Diego, CA) by using the Phagotest® kit (Orpegen Pharma, Heildelberg, Germany). For cytotoxic activity, peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Hypaque gradients (Lymphoprep, Nycomed, Oslo, Norway), counted and adjusted to 5x106 cells/mL. Then, natural killer (NK) cell activity was determined by specific target lysis of labelled K562 cells by flow cytometry, using the NKtest® kit (Orpegen Pharma).
Levels of serum IL-10, TNF-α, IL-8, IL-17, and IL-12 were quantified using a multiplex immunoassay (Cytometric Bead Array, CBA, BD Biosciences) by flow cytometry. The concentration of transforming growth factor (TGF)-β was determined by ELISA (BD OptEIATM, BD Biosciences).
Statistical analyses
Results were analyzed using the SPSS software (SPSS Inc. Chicago, USA). Goodness of fit to normal distribution was investigated with the Kolmogorov-Smirnov test. Significant differences in mean of nutritional and immunological parameters by age group were tested by using generalized linear models using gender and energy as covariates. Pearson s correlation coefficient analyses were conducted between immune variables and lipid and FA intake. Linear regression analyses were adjusted for gender. FA intake was expressed as percentage of total energy intake. Statistical parameter presented is (standardized regression coefficient). Differences were considered significant at P < 0.05 level.
Results
Table I shows the intake of protein, carbohydrates, fiber and lipids in both volunteer groups. The elderly group had a higher consumption of carbohydrates and a lower lipid intake than the middle-age group (table I). However, whilst MUFA intake was significantly lower in the elderly group the contrary was true for PUFA intake.
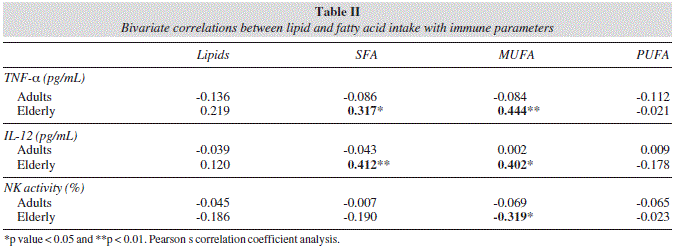
When Pearson s correlation coefficient between diet and immune variables was analyzed, no meaningful correlation was found for the adult group. However, whilst no correlations were observed in the elderly for TGF-β, IL8, IL-17, IL-10 or phagocytic activity (data not shown), some significant correlations were found for TNF-α, IL-12 and NK activity (table II). SFA and MUFA intake in elderly was positively correlated with TNF-α (r = 0.317 and 0.444, respectively) and IL-12 (r = 0.412 and 0.402, respectively). Additionally, MUFA intake was inversely correlated with NK cytotoxic activity (r = -0.319). Moreover, when the elderly group was classified according to the compliance with the current dietary recommendations for MUFA (> 15% of energy intake), it was found that elderly fulfilling the recommendation (n = 11) presented significantly higher plasma concentration of the pro-inflammatory cytokines IL-12 and TNF-α than those with intakes below the recommendation value (n = 29) (IL-12: 14.2 ± 12.9 vs. 2.9 ± 5.0 pg/mL and TNF-α: 12.0 ± 12.8 vs. 1.5 ± 5.7 pg/mL, respectively), whilst the contrary was true for NK activity (32 ± 20% vs. 50 ± 20%).
To further assess these associations, the relationships between fatty acids and preselected immune parameters were analyzed by linear regression analysis (table III). No statistically significant effects were found for total lipids or SFA. However, intake of MUFAs was found to be associated with IL-12 and TNF-α serum concentration in elderly. PUFA intake was inversely related to levels of IL-12, however, it did not explain the variations in serum TNF-α. On the other hand, no significant associations between total lipids or FA intake and NK cell activity were found. These associations were independent from gender.
Discussion
It is known that ageing is related with changes in the immune system,5,6 a phenomena commonly called "immunosenescence". This points out at the need of developing strategies to counteract these changes and restoring the immune parameters in elderly.
In this study a detailed dietary assessment was carried out and associations between dietary FA and different immune variables were studied. Several authors have suggested that certain dietary FA, particularly PUFA, play an important role on the health of the individual, having anti-inflammatory properties.14 However, whilst extensive research has been conducted on PUFA, less attention has been paid to MUFA, which have been often used as placebo in studies assessing the effects of PUFA on immune function.11 Nevertheless, MUFA may not be as immunologically neutral as previously expected and their use as placebo can be questioned.11,15 The positive association between MUFA intake and some pro-inflammatory cytokines (IL-12 and TNF-α) observed in our elderly population appears to emphasise this observation. This association between MUFA intake and pro-inflammatory cytokines was not confirmed in middle-age subjects, which, however, had a higher contribution of MUFA to energy intake, suggesting that either there is a threshold level for the effect of MUFAs on immune system or other factors related with senescence may also play a role.
It is widely known that malnutrition induces an attenuation of immune functions and it may be an important confounding factor in studies associating age with decreased immune response.2 Nevertheless, the elderly cohort under study had an adequate energy and protein intake, which was comparable to that of the middle-age group. Therefore, the different behavior observed between groups seems to be associated with age but does not appear to be a cumulative effect with nutritional status.
Despite the limited sample size our results emphasize the relationship between FA intake and inflammatory status at senescence. We observed that elderly fulfilling the current dietary MUFA recommendation presented higher plasma concentration of pro-inflammatory cytokines than those with intakes below the recommended level. These observations together with the positive correlation between these cytokines and MUFA intake in elderly subjects, which may exacerbate that inflammatory status, appear to indicate the need for reassessing the validity of the current general FA intake recommendations in the elderly population.
References
1. Christensen K, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009; 374: 1196-1208. [ Links ]
2. Mazari L, Lesourd BM. Nutritional influences on immune response in healthy aged persons. Mech Ageing Dev 1998; 104: 25-40. [ Links ]
3. Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S, Deng Y. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol 2007; 42: 719-732. [ Links ]
4. Candore G, Balistreri CR, Colonna-Romano G, Grimaldi MP, Lio D, Listi F, Scola L, Vasto S, Caruso C. Immunosenescence and Anti-Immunosenescence Therapies: The Case of Probiotics. Rejuvenation Res 2008; 11: 425-432. [ Links ]
5. Candore G, Caruso C, Jirillo E, Magrone T, Vasto S. Low Grade Inflamation as a Common Pathogenic Denominator in Age-Related Diseases: Novel Drug Targets for Anti-Ageing Strategies and Succesfully Ageing Achievement. Curr Pharma Des 2010; 16: 584-596. [ Links ]
6. Vallejo AN. Immunological hurdles of ageing: Indispensable research on the human model. Ageing Res Rev 2011; 10: 315-318. [ Links ]
7. Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res 2002; 43: 445-452. [ Links ]
8. James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 2000; 71: 343S-348S. [ Links ]
9. Mesa García MD, Aguilera García CM, Gil Hernández A. Importancia de los lípidos en el tratamiento nutricional de patologías de base inflamatoria. Nutr Hosp 2006; 21 (Suppl. 2):30-43. [ Links ]
10. Virella G, Fourspring K, Hyman B. Immunosuppressive effects of fish oil in normal human volunteers: correlation with the in vitro effects of eicosapentanoic acid on human lymphocytes. Clin Immunol Immunopathol 1991; 61: 161-176. [ Links ]
11. Yaqoob P. Monounsaturated fats and immune function. Braz J Med Biol Res 1998; 31: 453-465. [ Links ]
12. Fetterman JW, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm 2009; 66: 1169-1179. [ Links ]
13. Andujar MM, Moreiras O. Tablas de Composición de Alimentos. CSIC 1994. [ Links ]
14. Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009; 91: 791-795. [ Links ]
15. Puertollano MA, Puertollano E, Alvarez de Cienfuegos G, de Pable NA. Aceite de oliva, sistema inmune e infección. Nutr Hosp 2010; 25: 1-8. [ Links ]
![]() Correspondence:
Correspondence:
Miguel Gueimonde.
Department of Microbiology and Biochemistry of Dairy Products.
Instituto de Productos Lácteos de Asturias (IPLA).
Paseo Río Linares, s/n.
33300 Villaviciosa. Asturias. Spain.
E-mail: mgueimonde@ipla.csic.es
Recibido: 18-IX-2012.
Aceptado: 23-X-2012.