Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.28 no.3 Madrid Mai./Jun. 2013
https://dx.doi.org/10.3305/nh.2013.28.3.6058
ORIGINAL
Morphometric analysis of small intestine of BALB/c mice in models developed for food allegy study
Análisis morfométrico de intestino delgado de ratones BALB/c en modelos desarrollados para el estudio de alergia alimentaria
Tatiana Coura Oliveira1, Maria do Carmo Gouveia Pelúzio2, Sérgio Luis Pinto da Matta3, José Mário da Silveira Mezêncio4 and Josefina Bressan5
1Mestre em Ciência da Nutrição pela Universidade Federal de Viçosa. Professor Adjunto da Fundação Comunitària de Ensino Superior de Itabira. Brasil.
2Doutora em Bioquímica e Imunologia. Professor Adjunto IV da Universidade Federal de Viçosa. Brasil.
3Doutor em Biologia Celular. Professor Associado da Universidade Federal de Viçosa. Brasil.
4Pós Doutor pela Plum Island Animal Disease Center. Professor Adjunto IV da Universidade Federal de Viçosa. Brasil.
5Doutora em Fisiología y Nutrición pelo Universidad de Navarra Pamplona Navarra. Espanha. Professor Associado III da Universidade Federal de Viçosa. Brasil.
ABSTRACT
Although some animal models of food allergy in have already have been described, none of them uses the allergen in the animals' diet. This work describes the comparison between two developed models of food allergy in BALB/c mice, based in the administration of the allergen in the diet or by intragastric way. The experiment last for 28 days and the animals had been sensitized by means of subcutaneous injection in 1st and 14th days with in natura extract milk, bovine extract meat or frog extract meat. The experimental model that uses the allergen in the unbroken form presented morphometric alterations when compared with the one that used the heat treat allergen. It was noticed the existence of some more resistant proteins than others related to the denaturation, once compared the results of the two models; the differences had been more prominent for the milk and frog allergens. These results confirm the epidemiologic data of allergy incidence in the world's population.
Key words: Morphometry. Protein. Food allergy.
RESUMEN
Aunque algunos modelos animales para estudio in vivo de alergia alimentaria hayan sido descriptos, ninguno de ellos utiliza el alergeno en la dieta de los animales. Este trabajo describe la comparación entre dos modelos experimentales de alergia alimentaria desarrollados en los ratones BALB/c, inducida por la administración del alergeno en la dieta o por la vía intragastrica. El experimento fue desarrollado por un período de 28 días y los animales fueron sensibilizados por inyección subcutánea en el 1o y 14o días con extracto de leche in natura, extracto de carne de buey o extracto de carne de rana. El modelo experimental que recibió el alergeno intacto presentó las alteraciones morfométricas más evidentes cuando fueron comparadas con los que recibió el alergeno tratado térmicamente. Se evidenció la presencia de proteínas más resistentes que otras en lo que se refiere a la desnaturación, una vez que cuando fueron comparados los dos modelos, las diferencias fueron más claras para los alergenos de la leche y de la carne de rana. Estos resultados confirman los datos epidemiologicos de incidencia de alergia en la población mundial.
Palabras clave: Morfometría. Proteína. Alergia alimentaria.
Abbreviations
IgE: Imunoglobulin E.
kDa: Kilodaltons.
Th2: T helper cells type 2.
ECP: Extracellular release of cationic proteins.
UFV: Universidade Federal de Vigosa.
CD: Diet control.
AIN-93G: Semipurified diet standard for rodents.
LTT: Milk.
RTT: Frog meat.
BTT: Bovine meat.
CG: Gavage control.
GGL: Sensitized with milk protein.
GGR: Sensitized with frog protein.
GGB: Sensitized with bovine protein.
TT: Heat treatment.
Al(OH)3: Aluminium hydroxide.
AV: Villus height.
PC: Crypt depth.
LV: Villus width.
AE: Epithelium height.
MM: Muscle thickening of mucosa.
MI: Internal circular muscle thickening.
ME: External circular muscle thickening.
PT: Prick test.
BPCT: Blind placebo-controlled trial.
GT: Gastrointestinal tract.
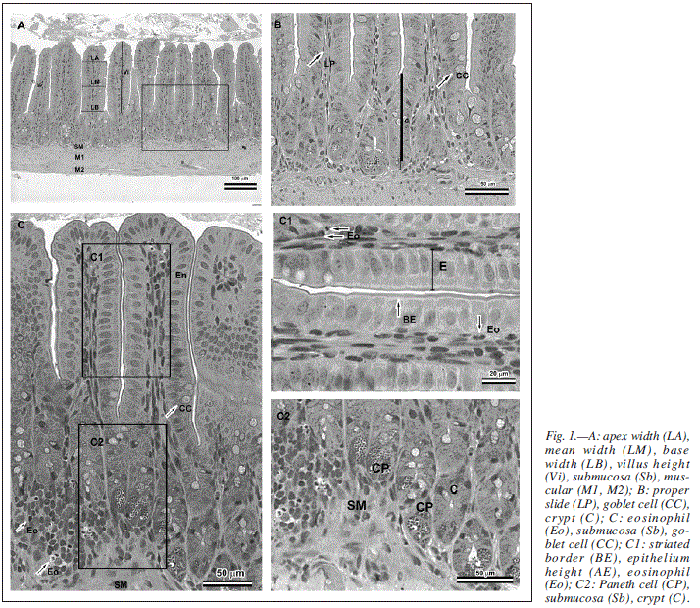
LA: Apex width.
LM: Mean width.
LB: Base width.
Vi: Villus height.
Sb: Submucosa.
Ml, M2: Muscular.
LP: Proper slide.
CC: Goblet cell.
C: Crypt.
Eo: Eosinophil.
Sb: Submucosa.
BE: Striated border.
AE: Epithelium height.
Eo: Eosinophil.
CP: Paneth cell.
Introduction
Allergy is essentially an inflammatory illness and the most common clinic manifestations linked to food allergy are skin related, mainly atopic eczema, and gastrointestinal mediated or not by IgE.1 Food allergy is characterized by a response of the immune system, mainly present in the gastrointestinal mucosa, to antigens orally ingested. Most of food allergens are low molecular weight proteins, ranging from 10 to 70 kDa, being the majority hydrosoluble and heat-resistant.2
At the same time that enterocytes are responsible for nutrients absorption, in the mucosa of the small intestine occurs most of the contact with antigenic materials in the gastrointestinal tract.3 Several defense mechanisms give to the gastrointestinal mucosa a complex structure that functions by using physiological and cellular factors to prevent antigens penetration. Its physical barrier is composed of enterocytes connected by junctional complex constituted by occlusive, adherence and communicating joints, covered by mucus. Mucus is secreted by goblet cells and is consisted basically of mucins with a great quantity of glycoproteins.4 Paneth cells also have an important role in the defense against microorganisms and allergens, since they produce polypeptides such as lysozymes and growth factors in lumen, which help in the protection process of the mucosa.5 As a consequence of the constant and great quantity of antigenic excitation factors, the intestine mucosa has the largest lymphoid complex of the body and large proportion of activated lymphocytes.6
During the food born allergic inflammation, in addition to T lymphocyte, other two cells seem to play an important role: eosinophil and mast. Eosinophils and masts are the main cells for immune response in the small intestine, considering Th2 cell the process coordinator. The main consequence of mast activation is the release of histamine and other mediators responsible for the acute status of allergic reaction. Activating eosinophils stimulates the extracellular release of cationic proteins (ECP) with potent cytotoxic action, and it is believed that they play an important role in the development of subacute and chronic symptoms of allergy.7 In consequence of its intense activity, there is a dynamic process of cellular proliferation, differentiation and death in the small intestine. In the crypts, there is cellular proliferation and migration towards the villi top.4 Several authors have reported that, in addition to a higher recruitment of activate immune cells, an early allergic sensitization can result in changes in the intestinal morphology.8,9,10
In animals, some studies developed with swines have shown a correlation between possible sensitization and changes in the intestinal morphology.3,11 Usually, the studies focus on different protein sources administered to animals soon after weaning.3 Therefore, the analysis of morphometric parameters of the intestinal mucosa can show situations of injury and local inflammation by modification of histological conformation of these areas.
It was an objective in this study to perform the morphometric analysis of the small intestine of BALB/c mice, subcutaneously sensitized, which later received the heat-treated allergen by diet or gavage, in its full form.
Material and methods
Animals
48 BALB/c mice of both sexes were used, with 7 weeks of age and mean weight of 20 ± 1.48 g, from the Animal Center of Health and Biological Sciences Center of UFV.
The animals were divided in two experimental groups. The first (table I) was composed by four subgroups: diet control (CD), with non-sensitized animals that received semipurified diet standard for rodents (AIN-93G)12 and three subgroups called "positive controls" with animals sensitized with milk proteins (LTT), frog meat (RTT) and bovine meat (BTT) in natura, which received AIN-93 diets modified in protein composition according to sensitization. The second (table I) was also composed by four subgroups: gavage control (CG), with non-sensitized animals that received AIN-93G12 diet and gavage with distilled water, and three other subgroups called "positive controls" with animals sensitized with milk (GGL), frog meat (GGR) and bovine meat (GGB) in natura extracts, which received AIN-93 diet and gavage of allergen extract.
During the experiment, the animals were kept in collective cages, separated according to diet and sex, in environment with controlled temperature (22o C) and light/dark 12-hour cycle, receiving food and water ad libitum.
Preparation of diets
Diets were prepared based on the AIN-93G12 diet with modification in the type of protein being offered, according to group sensitization (table II). Skimmed powered milk and bovine meat were purchased in the local trade, since frog meat originated from the Frog Farm of UFV.
Meat samples, both bovine and frog, were processed in order to simulate the domestic heat treatment (TT), in the Foods Experimental Study Laboratory of the Departamento de Nutricao e Saúde. Dry heat was applied, under temperature of 95o C for 15 minutes and later dehydration in oven with airflow at 65o C for 4 hours. For milk, no heat treatments were used additional to industrial processing.
All ingredients were weighed in semi-analytical balance. Diets were weekly prepared, identified and stored at 4o C until the distribution moment.
Sensitization protocol
The experiment lasted 28 days from first day (D1). Sensitization occurred by subcutaneous injection of 1 mg of allergen, in extract form, with 1 mg of Al(OH)3 as adjuvant. Sensitization occurred in two moments: D1 and D14, with the use of the same protocol.
Preparation of the extract for sensitization and gavage
To prepare the extract of meats, 100 g of bovine meat and 100 g of frog meat were used. Firstly, mechanical grinding was performed by a food multiprocessor. Next, 50 mL of distilled water was added to the chopped meat and the mix was manually macerated for 1 minute. The product was strained twice, in sterilized gauze to eliminate solid residues. The quantity of protein of the resulting extract was analyzed, and adequate by dilution to meet the protein specification for sensitization and gavage.
Each animal received during the experiment two doses of 0.5 mL of the extract containing 1 mg of the allergen protein, by gavage, according to the received diet and sensitization. Doses were administered in the 8th and 16th days of experiment.
Material collection
On the 28th day, animals were euthanized, blood samples were collected from the abdominal aorta and stored; fragments of the 3 small intestine sections were collected and fixed in buffered formaldehyde for 24 hours and histologically processed for morphometric analysis.
Histological preparations were performed in the Structural Biology Laboratory of Departamento de Biologia Geral (UFV). Duodenum, jejunum and ileum, after dehydration in ethanol series and inclusion in resin (Historesin® -Leica) were sectioned in rotating microtome (RM 2155-Leica), transversely and longitudinally, in 2 pm thickening, and stained with hematoxylin and eosin.
After obtaining the images in photomicroscope (AX-70 Olympus), histological preparations were submitted to morphometric analysis with aid of software for images analysis (Image Pro Plus 4.0-Media Cybernetics®). Analyzed morphometric parameters were identified in figure 1.
Later, eosinophil count was performed in the histological slides in three distinct areas of each intestinal section, in a total of 5.7 mm2 assessed per small intestine segment of each animal.
Regarding morphometry, the measured values in animals for parameters villus height (AV), crypt depth (PC), villus width (LV), epithelium height (AE), muscle thickening of mucosa (MM), internal circular muscle thickening (MI) and external circular muscle thickening (ME).
Statistic analyses
Data were statistically analyzed by using the Statistics software for variance analysis, with the use of Duncan test of averages or t Student test, whenever adequate, with a 5% significance level.
Results and discussion
Concerning the food consumption, there was no statistically significant difference (p > 0.05) among the groups in the experimental model in which mice received the allergen through diet. It is evidenced, however, decrease in food consumption (fig. 2) in the days following sensitization of the animals, what was expected since the immune response is locally formed and could decrease the appetite of animals.
Few works discuss the food consumption since the allergen is usually conveyed in drinking water, not diet. In these cases, weight loss in consequence of dehydration is reported, thus confirming a lower material consumption that is conveyed to the allergen, whether in food or drink.13
The animals that received gavage with in natura allergen extract showed a marked decrease in food consumption (fig. 3) after the first sensitization when compared to the second sensitization. A statistically significant difference (p < 0.05) between the group CG and other groups and among animals of groups GGL and GGB, between groups GGR and GGB.
One possible rationale for the differences found in food consumption data of groups GGL and GGB could be the probability of larger allergenicity of milk versus bovine meat, since gavage had these allergens.14,15
Another point that should be highlighted is the fact that in the extract administered by gavage proteins were intact, a state that gives greater allergenic power to the protein fractions.16 Host and Samuelson17 investigated the allergenic potential of in natura milk, pasteurized milk at 75o C for 15 seconds, and pasteurized and homogenized at 60o C in children. All of them showed positivity for prick test (TP) and blind placebo-controlled trial (BPCT) with elevated trend to allergenicity, including for processed samples. Contrary to the results presented by Sampson and MacCaskill18, who found positivity in TP for bovine meat in 15.9% in known atopic individuals, although after BPCT only 1.8% were confirmed as allergic to bovine meat.
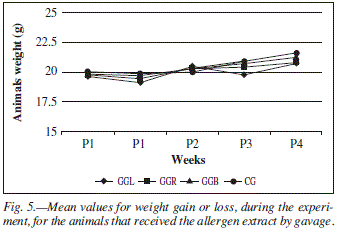
Concerning weight, there was no difference among groups that received the allergen by diet (fig. 4) despite the different values for weight gain and loss found during the experiment. The weight of the animals in the groups in which allergen was administered by gavage (fig. 5) had no statistically significant difference.
To assess the action of the different allergen and administration, leukocytes global and differential count were performed. Global count had no significant results (p > 0.05) between treatments and they were all within normal range for the species.
Concerning eosinophils, the following values were found: 0.07; 0.06; 0.05 and 0.02 x 103 cels/ml, respectively for LTT, RTT, BTT and CD. For animals that received gavage, the values were 0.21; 0.06; 0.07 and 0.01 x 103 cels/ml for GGL, GGR, GGB and CG, respectively. Normal range varied from 0.0 to 0.38 x 103 cels/ml and, therefore, despite differences, the values were within normal range.19
In the eosinophils count in small intestine, animals that received heat-treated allergen had the mean 18 ± 9.28; 18 ± 11.06; 16 ± 9.26 and 11 ± 3.81 for the groups LTT, RTT, BTT and CD, respectively. The count performed in the histological preparations of animals that received allergen by gavage, the means found were 28 ± 16.88; 20 ± 7.54; 13 ± 8.92 and 15 ± 7.6 eosinophils for the groups GGL, GGR, GGB and CG, respectively. No statistically significant differences in any presented results were found.
Analysis performed by rectosigmoidoscopy in individuals with allergy to cow milk, swollen and hyperemic mucosa is evidenced20 and microscopy usually shows the preserved architecture of crypts and enterocytes, but with strong eosinophilia and presence of intraepithelial macrophages, neutrophils and lymphocytes.21
Eosinophils are normally found throughout the gastrointestinal tract (GT), except in the esophagus of young patients. In case of biopsies of the GI it must taken into consideration if the number of eosinophils is significantly higher than the normal density for a certain anatomical site. Criteria for eosinophilia of GI are varied, but generally the presence of eosinophil in the esophagus of young patients is considered abnormal. Children's stomach usually presents a low density of eosinophils in the mucosa, with superior concentrations in the small intestine. Some pathologies can generate significant recruitment of eosinophils in the GI tract and are called eosinophilic gastrointestinal disorders, being defined as disorders that primarily affect the GI tract with inflammations high in eosinophils in the absence of known causes for eosinophilia. At least a subset of patients that present this type of pathology seem to have allergic illnesses, with intermediate characteristics between food allergy mediated by IgE and hypersensitivity mediated by cells.22
There are animal models for eosinophilic gastroenteritis, there they indicate that, associated to eosinophilia, an increase in masts markers coexist, indicating an association of these two cellular types in the pathophysiology of the eosinophilic gastroenteritis.22,23,24 In some of these models, especially those developed with mice, interleukin 5 (IL5) release is pointed as the regulating key of eosinophilic accumulation in the GI.21 Interestingly, there are also reports of eosinophilic esophagitis in allergy models in which the antigen administration is intranasally.25
When compared, morphometric variables analyzed for allergen types in the different segments of small intestine for animals that received the allergen by diet, it was found in the duodenum statistic difference between AV of group LTT animals (fig. 6) and groups BTT and CD animals. Difference (p < 0.05) was also found between group RTT and groups BTT and CD. Additionally, there was statistically significant difference (p < 0.05) in the jejunum for measured values for AV between groups LTT and CD.
Such findings prove with data published by Scandolera et al.3 which compare different protein sources used in swine ration when weaning, being that for all treatments similar deleterious effect was found over the morphology of the intestinal mucosa, and none of the used protein sources was able to minimize such effects in the animals.
Regarding values for PC, no statistic difference was found for values measured in duodenum or ileum of animals that received the allergen by diet (fig. 7). In the jejunum a difference for PC was found between groups LTT and BTT.
When there is cellular renewal in the intestinal mucosa, there is hyperplasia in crypt cells and shift towards the villus.26 Therefore, it was expected a significant increase in the crypt depth in animals that were sensitized and consumed milk protein, because it has lactoglobulin, protein fraction with known allergenicity when compared to others in the literature.27
A good villus height/crypt depth ratio occurs when villi are high and crypts are little deep, providing better absorption of nutrients.28
Considering that the basic form of villus is similar to a conical structure, the increase in its width could indicate change of its elongated form to flat.26 Therefore, width increase of villus tends to happen in groups that evidenced statistically significant differences of villus height.
When the LV parameter is assessed (fig. 8), a statistically significant difference (p < 0.05) in the duodenum was found between group LTT when compared to group BTT. Concerning the parameter AE, a statistically significant difference was found only in the jejunum of groups BTT and CD animals.
For values measured for MM (fig. 9) a statistic difference (p < 0.05) was found in the duodenum between groups BTT and CD. In the jejunum, a statistically significant difference was found in the group LTT when compared to groups BTT and RTT, and in the groups RTT and BTT when compared to group CD. For the ileum a statistically significant difference was found between groups BTT and CD.
For MI, a difference (p < 0.05) was found in values measured in the duodenum between the group LTT when compared to groups RTT and BTT, and in groups BTT and RTT when compared to the group CD; in the jejunum the difference was found between the group LTT when compared to groups BTT and CD; in the ileum no significant difference was found. Concerning the values measured for ME, no statistic difference was found (fig. 10).
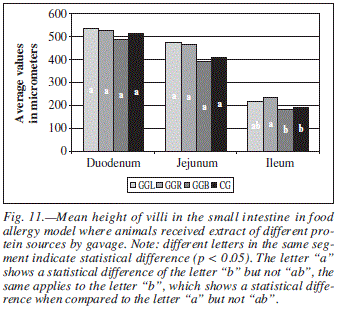
For parameter AV (fig. 11) measured in animals that received the allergen by gavage, significant differences (p < 0.05) were found only in the ileum for the group GGR when compared to groups GGB and CG.
Concerning the variable PC (fig. 12), statistic difference (p < 0.05) was found in the ileum among animals of groups GGL and GGR and among the group GGR when compared to groups GGB and CG. Crypts depth is directly related to an increase in cellular proliferation, which tends to happen in an exacerbated form in inflammation periods or intestinal mucosa injury.9
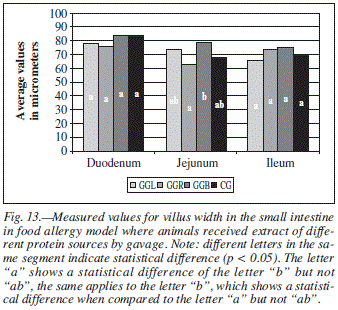
When the groups were compared regarding to LV, difference was found only in the jejunum for groups GGR and GGB (fig. 13).
For variable MM, there was difference (p < 0.05) between groups GGB and CG. For variable MI, values measured in the duodenum showed difference when compared to groups GGL and GGR, in the jejunum when compared to group GGL when compared to groups GGR and CG and between GGB and CG. There was also difference for ME values in the duodenum between the group GGL when compared to groups GGR and CG and between groups GGB and CG.
There was also a comparison among morphometric variables in the different experimental models used, by type of allergen in the investigated segments in the small intestine.
Statistically significant difference in villus height and width and crypt depth in the duodenum when the allergen used was milk was found. In table III it can be clearly seen that the measured value for AV of group GGL is approximately 18% lower than the measured value in group LTT, whereas the mean value for PC is approximately 12% higher than the measured value for the same parameter in the group LTT. As previously discussed, such finding can be a consequence of the lactoglobulin presence in the milk extract, a protein fraction acknowledged in the literature with significant antigenic power, especially when it is natively administered.29
Still with milk as allergen, statistically significant difference was found for the epithelium height in the jejunum, 32.4496 ± 3.15 mm and 26.9036 ± 2.17 mm, respectively for animals that received protein from heat-treated die and gavage, respectively. Results found in this experiment reinforce epidemiological data discussed in the literature concerning the incidence of food allergy in world population, since the allergy to cow milk has larger frequency when compared to allergy to cow meat in the general population.30
When the allergen used was frog extract, statistically significant difference (p < 0.05) was found in the duodenum for parameters villus height and width and epithelium height (table III). Group RTT had a value for AV approximately 24% higher than that presented by group GGR.
Once again it was evidenced that proteins natively administered have larger possibility of sensitize and cause deleterious effects in larger proportion than when administered post-heat processing.
When the jejunum was analyzed (table IV), we found difference for frog allergen for variables: villus width and internal muscle width.
For the ileum segment, statistic difference was found in measured values for crypt depth and internal muscular (table IV). In this case, a simultaneous decrease of mean villus height and increase of mean crypts depth of animals that received gavage must be stressed, clearly indicating a hyperplasic process.
When analyzing the usage of bovine extract as allergen, we find in the duodenum statistically significant difference for villus height 587.82 ± 31.63 mm and 512.11 ± 15.51 mm for heat treatment and gavage, respectively. No other parameter showed change.
Controls groups, CD and CG, had statistically significant difference (p < 0.05) for AV, LV, AE and MI in duodenum (table V) and for AE in the jejunum, with 32.98 ± 03.04 mm in the group that received heat-treated allergen, and 29.01 ± 2.75 mm in the group that received gavage.
These data prove that the fact of gavage use can contribute for the process of intestinal morphologic change, since the intragastric administration is more deleterious than orally normal consumption.
Conclusion
It was evidenced with the comparison between morphometric parameters of experimental models for the study of food allergy that heat treatment is efficient in reducing the allergenic potential of proteins, since it provided less morphometric changes in the small intestine of animals that received allergen in the diet when compared to those that received allergen by gavage. It also evidenced the existence of some more resistant proteins than others related to denaturation, once compared the results of the two models, the differences mainly for villus height and crypt depth had been more prominent for milk and frog meat extracts.
Regarding frog meat, although it had an intermediate position to milk and bovine meat concerning morphometric changes for nearly all analyzed variables, it is too soon to state that its use is safe, especially in individuals with genetic susceptibility. Even in the literature, data about its use replacing other protein sources are controversial.
The use alternative meats by allergic individuals must be cautiously analyzed, since no protein can be considered hypoallergenic. Also, there is the possibility of crossed reactivity between foods.
References
1. Mofidi S. Nutritional management of pediatric food hypersensitivity. Allergy 1999; 54: 352-57. [ Links ]
2. Nowak-Wegrzyn A. Future approaches to food allergy. Pediatrics 2003; 111 (6): 1672-80. [ Links ]
3. Scandolera AJ, Thomaz MC, Kronka RN, Fraga AL, Budiño FEL, Huaynate RAR, Ruiz US, Cristani J. Efeitos de fontes protéicas na dieta sobre a morfologia intestinal e o desenvolvimiento pancreático de leitóes recém-desmamados. Rev Bras Zootec 2005; 34 (6): 1447-85. [ Links ]
4. Mandir N, Fitzgerald AJ, Goodlad RA. Differences in the effects of age on intestinal proliferation, crypt fission and apoptosis on the small intestine and the colon of the rat. Int J Exp Path. 2005; 86:125-30. [ Links ]
5. Verburg M, Renes IB, Meijer HP, Taminiau JAJ, Buller HA, Einerhand AWC, Dekker J. Selective sparing of goblet cells and Paneth cells in the intestine of methotrexate-treated rats. Am. J. Physiol. Gastrointest. Liver Physil 2000; 279: 1037-47. [ Links ]
6. Bischoff SC, Mayer J, Nguyen Q, Stolte M, Manns MP. Immuno-histological assessment of intestinal eosinophil activation in patientes with eosinophilic gastroenteritis and inflammatory bowel disease. Amer J Gastroenterol 1999; 94 (12): 3521-29. [ Links ]
7. Cordle TC, Winship TR, Schaller JP, Thomas DJ, Buck RH, Ostrom KM, Jacobs JR, Blatter MM, Cho S, Gooch WM, Pickering LK. Immune status of infants fed soy-based formulas with or without added nucleotides for 1 year: Part 2: Immune cell populations. J Pediatr Gastroenterol Nutr 2002; 34 (2): 145-53. [ Links ]
8. Komori H, Meehan TF, Havran WL. Epithelial and mucosal T cells. Curr Opin Immunol 2006; 18: 534-38. [ Links ]
9. Cummins AG, Jones BJ, Thompson FM. Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig Dis Scie 2006; 51 (4): 718-23. [ Links ]
10. Li DF, Nelssen JL, Reddy PG. Interrelationship between hyper-sensitivity to soybean proteins and growth performance in early weaned pigs. J Anim Scie 1991; 69 (8): 4062-69. [ Links ]
11. Boratto AJ, Lopes DC, Oliveira RFM, Albino LFT, Sa LM, Oliveira GA. Uso de antibiotico, de probiotico e de homeopatia, em frangos de corte criados em ambiente de conforto, inoculados ou não com Escherichia coli. Rev Bras Zootec 2004; 33 (6): 1477-85. [ Links ]
12. Reeves PG, Nielsen FH, Fahey GC. AIN-93 Purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 939-51. [ Links ]
13. Saldanha JCS, Gargiulo DL, Silva SS, Carmo-Pinto FH, Andrade MC, Alvarez-Leite JI, Teixeira MM, Cara DC. A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Braz J Med Biol Res 2004; 37 (6): 809-15. [ Links ]
14. Motrich RD, Gottero C, Rezzonico Jr C, Rezzonico C, Riera CM, Rivero V. Cow's milk stimulated lymphocyte proliferation and TNF-α secretion in hypersensitivity to cow's milk protein. Clin Immunol 2003; 109: 203-11. [ Links ]
15. Fiocchi A, Restani P, Riva E. Beef allergy in children. Nutrition 2000; 16: 454-57. [ Links ]
16. Besler M, Steinhart H, Paschke A. Stability of food allergens and allergenicity of processed foods. J Chromatogr 2001; 756: 207-28. [ Links ]
17. Host A, Samuelsson EG. Allergic reactions to raw, pasteurized, and homogenized/pasteurized cow milk: a comparison. Allergy 1988; 43: 113-7. [ Links ]
18. Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatric 1985; 107: 669-75. [ Links ]
19. Suckow MA, Danneman P, Brayton C. The laboratory mouse. USA: CRC Press; 2001. [ Links ]
20. Iacono G, Carroccio A, Cavatario F, Montalto G, Cantarero MD, Notarbartolo A. Chronic constipation as a symptom of cow milk allergy. J Pediatr 1995; 126: 34-9. [ Links ]
21. Machida HM, Smith AG, Gall DG, Trevenen C, Scott RB. Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr 1994; 19: 22-6. [ Links ]
22. Lampinen M, Carlson M, Sangfelt P, Taha Y, Thorn M, Loof L, Raab Y, Venge P. IL-5 and TNFα participate in recruitament of eosinophils to intestinal mucosa in ulcerative colitis. Dig Dis Scie 2001; 46 (9): 2004-09. [ Links ]
23. Chehade M, Berin MC, Sampson HA. Intestinal mast cells are increased in a mouse model of eosinophilic gastroenteritis following oral allergen challenge. Clin Immunol J Allergy 2004; 113 (2): 90-5. [ Links ]
24. Teixeira MM, Talvani A, Tafuri Wl, Lukacs NW, Hellewell PG. Eosinophil recruitment into sites of delayed-type hypersensitivity reactions in mice. J Leukoc Biol 2001; 69 (3): 353-60. [ Links ]
25. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107: 83-90. [ Links ]
26. Schneeman BO. Gastrintestinal physiology and functions. Brit J Nutr 2002; 88 (2):159-63. [ Links ]
27. Rancé F, Kanny G, Dutau, G, Moneret-Vautrin, DA. Food hypersensitivity in children: clinical aspects and distribuition of allergens. Pediatr Allergy Immunol 1999; 10: 33-8. [ Links ]
28. Abreu MLT, Leão MI, Matta SLP. Alterações morfológicas intestinais em leitões desmamados precocemente alimentados com níveis crescentes de farelo de soja. VI Congresso Internacional de Medicina Veterinària em Lingua Portuguesa; 1993 Dezembro 06-10; Salvador (BA) pp. 394-7. [ Links ]
29. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol 1996; 14: 1269-73. [ Links ]
30. Beretta B, Conti A, Fiocchi A. Antigenic determinants of bovine serum albumin. Int Arch Allergy Immunol 2001; 126: 188-95. [ Links ]
![]() Correspondence:
Correspondence:
Tatiana Coura Oliveira.
Fundação Comunitária de Ensino Superior de Itabira.
Rua Venâncio Augusto Gomes, 50 - Prédio Areão - Bairro Major
Lage de Cima.
CEP: 35900-842 - Itabira/MG.
E-mail: contato.tatiana@gmail.com
Recibido: 14-VII-2012.
Aceptado: 24-VIII-2012.