Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.3 Madrid may./jun. 2013
https://dx.doi.org/10.3305/nh.2013.28.3.5976
ORIGINAL
Influences of different thermal processings in milk, bovine meat and frog protein structure
Influencia del tratamiento térmico en la estructura proteíca de leche, carne y rana
Tatiana Coura Oliveira1, Samuel Lopes Lima2 and Josefina Bressan3
1Mestrado en Ciência da Nutrição pela Universidade Federal de Viçosa (Brasil). Professor Adjunto da Fundação Comunitària de Ensino Superior de Itabira (Brazil).
2Doutorado en Ecologia e Recursos Naturais pela Universidade Federal de São Carlos (Brasil). Supervisor Técnico Científico do Ranaville Agroindustrias Ltda.
3Doutorado em Fisiologia y Nutrición pelo Universidad de Navarra. Pamplona. España. Profesor Associado III da Universidade Fedral de Viçosa. Brasil.
ABSTRACT
Several studies have associated the digestibility of proteins to its imunogenic potential. Though, it was objectified to evaluate the impact of the thermal processing with high and low temperatures on the proteins structure of three types of foods, by means of the digestibility in vitro and electroforesis en gel de poliacrilamida. The pasteurize was observed in such a way, firing 95 oC during 15 minutes, how much freeze dried causes qualitative and quantitative modifications of constituent proteins of the food. The most sensible proteins to the increasing thermal processing order were beef, frog meat, and the last, cow milk.
Key words: Food allergens. Thermal processing. Digestibility.
RESUMEN
Varios estudios han asociado la digestibilidad de las proteínas para su potencial inmunogénico. En este sentido, el objetivo fue evaluar el impacto del tratamiento térmico a temperaturas altas y bajas en la estructura de la proteína de los tres alimentos a través de la digestibilidad in vitro y la electroforesis en gel de poliacrilamida. Se observó que tanto la pasteurización, la cocción a 95 oC durante 15 minutos y liofilización dio modificaciones cualitativas y cuantitativas de los constituyentes de proteínas de los alimentos. Las proteínas más sensibles al tratamiento térmico en orden ascendente fueron carne de res, carne de rana y, finalmente, la leche de vaca.
Palabras clave: Alérgenos alimentarios. Tratamiento térmico. Digestibilidad.
Abbreviations
PAGE: Polyacrylamide gel electrophoresis.
IgE: Immunoglobulin type E.
PT: Prick test.
BPCT: Blind placebo-controlled trial.
BSA: Bovine serum albumin.
HT: Heat treatment.
% D: Digestibility percentage.
LB: Lysis buffer.
SB: Sample buffer.
kDa: Kilodaltons.
GMO: Genetically modified organisms
i.n. milk: In natura milk
HT milk: Powdered milk.
R frog: Raw frog meat.
HT Frog: Cooked frog meat.
R beef: Raw beef.
HT Beef: Cooked beef.
OSA: Ovine serum albumin.
MW: Molecular weight
Introduction
Thermal processing is used to improve the quality of food microbiological safety, either by eliminating micro-organisms or toxins or by improving the nutritional value which results from digestibility increase).1 Significant changes occur in the tertiary structure of proteins during heat treatment. The nature and extent of these changes depend on the temperature and duration of thermal processing, as well as the inherent protein characteristics and the physical-chemical conditions involved.1
Several allergens found in foods are heat-resistant and stable to digestion performed in the gastrointestinal tract, leading some researchers to correlate the allergenic potential of some foods to their stability to the action of proteolytic enzymes.1,2,3 In addition to denaturation, other covalent modifications due to heat or food storage can lead to change in food allergenicity. Some examples are lipid oxidation reactions or the direct oxidation caused by oxygen-reactive intermediates.4
Food-induced allergic reactions are responsible for a variety of symptoms involving the gastrointestinal, respiratory and skin systems, and can be caused by mechanisms whether mediated or not by immunoglobulin type E (IgE).5 Any type of food can cause an allergic reaction in the presence of genetic susceptibility, but in effect a small number of foods are actually responsible for most reactions. These include cow's milk, eggs, fish, seafood, peanut, soybean, wheat, beef, pork and some citrus fruits.5,6,7,8,9 Studies suggest that about 2% of adults worldwide have food hypersensitivity, 1% of which is food allergy itself; figures are generally higher for children under three years old, ranging between 6% and 8%.1,2
The application of heat treatment can usually reduce fresh fruit allergenicity easily, allowing the food industry to produce allergy-safe food.10 To assess the influence of heat treatment on allergy clinical reactivity, Fiocchi et al.11 compared the effects of domestic cooking and industrial processing using the prick test (PT) and the blind placebo-controlled trial (BPCT) in institutionalized children. In the first test, industrially processed meat extract was dissolved in glycerol (50%) and compared with raw, cooked and freeze-dried powdered beef extracts. Purified bovine serum albumin (BSA) was used as positive control; 10 children were positive for at least 03 of the items tested. In a second test, the same individuals participated in the BPCT for industrially processed beef steamed for 5 minutes at 100o C, lyophilized raw beef and purified BSA, where turkey meat was used as a placebo. The protocol used an initial dose of , which was doubled every 30 minutes (24, 48 and of test food or placebo) for 4 hours and discontinued when the first symptoms arose, or when there was a negative response after the eighth dose. Positive responses were found only for purified BSA in 50% of individuals, who manifested rhinitis, angioedema, urticaria and asthma, thus demonstrating that heat treatment is able to reduce protein allergenicity.
Sites of IgE binding to the protein allergen may consist of consecutive segments of the amino acid or different parts of the amino acid sequence held together by protein conformation, which are the so-called conformational antigenic determinants.12,13 Some antigenic determinants are accessible in native proteins and are lost when they are denatured; others are exposed when the protein unfolds; there are also some determinants arising from covalent modification caused by peptide bond breakdown.14 According to some researchers, peptide action is able to influence serum albumin allergenicity by cleaving amino acid sequences and turning an allergen into a non-allergenic protein.2
Low-temperature industrial processing can also modify food protein structure since the food protein structure between proteins and water is reduced.1 Freeze drying is the most commonly used method to prepare dehydrated proteins, which should have adequate stability in long storage periods at room temperature.7 Freeze drying basically involves three steps: freezing, primary drying and secondary drying. Freezing stops chemical reactions and possible biological activities in the sample. The previously frozen material is dried by sublimation followed by desorption, using low-temperature drying at reduced pressure.15,16
In this regard, this study aimed to evaluate the impact of high and low-temperature heat treatment on the protein structure of three foods by means of in vitro digestibility and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Material and methods
The samples analyzed were selected in order to be compared regarding their stability during thermal processing. An analysis was made of cooked, raw, and raw lyophilized frog meat and beef samples; and in natura, in natura lyophilized, pasteurized, and industrially-processed powdered cow's milk.
Frog meat is cited in the literature as a possible replacement protein source in diets for allergic individuals, despite the scarcity of studies addressing its use. 17, 18 Beef has a low incidence of allergy, whereas cow's milk has more than 25 different and potentially antigenic proteins. These include a- and b-lactoglobulins, and S1 and S2 a and k caseins, which are known to be allergenic when ingested by susceptible individuals. The incidence of cow's milk allergy in the pediatric population ranges from 0.5 to 7.5%.19,20
Sample collection and preparation
In natura and pasteurized milk was obtained from the Universidade Federal de Vigosa (UFV) Dairy Cooperative; frog meat came from the Frog Farm at UFV, while powder milk and beef were purchased from local traders.
The beef and frog meat samples were processed to simulate home heat treatment (HT) at the Laboratory of Experimental Study of Food of the Nutrition and Health Department under dry heat at a temperature of 95o C for 15 minutes. Subsequently, the samples for in vitro digestibility were submitted to dehydration in an oven at 65o C for 4 hours. For milk samples, only industrial processing was used.
Protein value
To determine nitrogen content, the samples were analyzed by the semi-micro Kjeldahl method in accordance with regulations of the Association of Official Analytical Chemists.21
In vitro digestibility
In vitro digestibility was evaluated with the method described by Hsu et al.22 according to which digestibility is characterized by pH decrease in the protein solution measured in the first 15 seconds and then at every minute for 10 minutes after the enzyme solution is added.
The samples were suspended in distilled water, of protein/mL, with final pH equal to 8, stirred au bain-marie at 37o C. For the hydrolysis of the prepared samples, we used 5 mL of enzyme solution containing 2.5 mg/mL trypsin and 1.6 mg/mL pancreatin.
Digestibility percentage (% D) was calculated with the equations described by Pires et al.23 originating from the correlation between values observed in in vitro analyses with in vivo experiments.
Polyacrylamide gel electrophoresis
In this procedure, samples of the following kinds of food underwent polyacrylamide gel electrophoresis (PAGE): frog meat and beef samples that were raw, raw lyophilized and dry-heated ( for 15 min); in natura, in natura lyophilized, pasteurized and powder cow's milk obtained by industrial processing. Electrophoresis was performed according to Laemmli24.
Solid samples were macerated in 200 mL of lysis buffer (LB) until completely dissolved, except for the lyophilized sample, which was suspended in 100 mL distilled and deionized water before maceration. The samples were then centrifuged (Centrifuge - Eppendorf) for 2 minutes at 14,000 rpm; the supernatant was removed for later use, while the liquid samples were added to distilled and deionized water.
Subsequently, an aliquot of 100 mL was taken from each previously prepared sample and 100 mL of twice-concentrated sample buffer (SB) was added. After a short homogenization treatment, the samples were boiled au bain-marie for 2 minutes, 10 mL of sample was applied in each "slott", and electrophoresis occurred at 10 mA for 17 hours. A standard marker for low molecular weight proteins (Mobitec®) was used, with extreme values of 116 kDa and 14 kDa.
Statistical analysis
The data were statistically analyzed with the Statistics software by analysis of variance using the Duncan means test or Student's t test, where appropriate, with a significance level of 5%.
Results and discussion
When protein content was compared in the studied samples (table I), statiscally significant difference was found between in natura samples and samples heat-treated by means of cooking and dehydration. However, no statistically significant difference was found for protein content when the samples were separated into two groups: heat-treated and unheated samples.
Digestibility is defined as the calculation of the percentage of proteins that are hydrolyzed by digestive enzymes and absorbed as amino acids, or any other compound nitrogenated by the human organism. Digestibility also determines the protein quality of a diet.23 Methods to determine in vitro digestibility are based on the digestion of a sample with proteolytic enzymes in standardized conditions. Protein digestibility has been routinely assessed in procedures aiming to investigate the safety of new proteins from genetically modified organisms (GMO). It is also crucial for research on the influence of heat treatment on the allergenic potential of several foods, among other applications.25
Figure 1 shows a more dramatic pH decrease until the second minute for all the samples, and then a slower drop until the tenth minute, which results from the fact that denatured proteins are more sensitive to the action of proteolytic enzymes. Thus, the breakdown of peptide bonds and hydrogen bonds tends to modify the pH of the medium because the load of acid amino acids is exposed. A cascade reaction is then initiated, since the proteins are sensitive to the pH of the solution where they are dissolved.
The results found for the in vitro digestion of the lyophilized and heat-treated in natura samples (fig. 2) showed no statiscally significant difference (p > 0.05). This result may be a consequence of the modifications made to proteins by both freeze drying and cooking. Proteins are known to denature, sometimes irreversibly, because of several events that affect their stability, such as heating, agitation, freezing, pH changes and beyond exposure to interfaces or denaturants.26 On the whole, the values obtained for digestibility percentage (fig. 2) ranged between 80%, for powdered milk, and 69%, for heat-treated beef.
Food processing can improve food taste and texture as well as inactivate antinutritional factors. However, it can also change the primary structure of proteins leading to the oxidation of sulfur-containing amino acids and cross-linking between peptides, which decreases the bioavailability of essential amino acids.1,4,27
Both heat treatment and long-term food storage can produce harmful effects on the nutritional quality of proteins. Changes in the nutritional value include a decrease in protein digestibility, a reduction in the bioavailability of lysine and other essential amino acids, and perhaps foster the production of substances which may be growth-inhibiting or toxic, for example, lysinoalanine. At least two mechanisms are involved in the decrease in protein quality: one of the amino acid side chains is blocked, and cross-linking occurs between peptide chains by means of condensation reactions.1,28,29
Still, the values obtained for in vitro digestibility (fig. 2) conform with the expectations for animal proteins, since the values obtained for in vitro digestibility analyses are usually lower than the ones found for protein quality analyses performed in experiments with animals.30
Restani et al.31 investigated different standards related to in vitro digestion of albumins in their allergenic potential, and discovered that 5 minutes after protease activity, there was a statiscally significant reduction in the number of positive prick tests performed for BSA and ovine serum albumin (OSA), when compared to the same test performed with proteins in their native state.
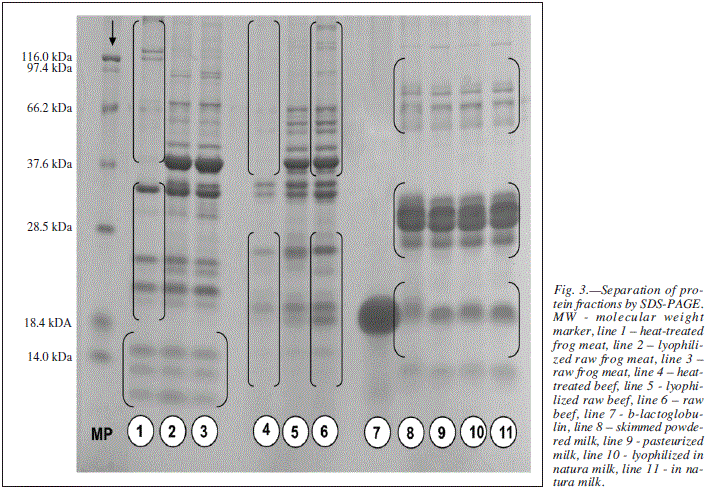
To perform an approximate calculation for molecular weight (MW) values of protein bands, the correlation between MW and the distance covered by the proteins of the marker was used by means of the equation y = -0.0699x .+ 2.1663.32
Figure 3 shows the electroforetic behavior of proteins in the beef and milk samples analyzed by PAGE according to the different heat treatments applied.
When frog meat and the different treatments applied to it are taken into account (lines 1, 2 and 3), it is observed that the low molecular weight proteins were apparently not cleaved and seem to have remained intact when cooked or lyophilized. All proteins smaller than 28 kDa remained stable either when cooked at 95o C for 15 minutes or lyophilized, and also when untreated.
Freeze drying can cause several structural changes in the protein spectrum. Recent studies with infrared spectroscopy have reported that problems related to lyophilization-induced freezing and dehydration can lead to the molecular unfolding of proteins.33 Protein drying during lyophilization usually causes α-helical structures to decrease and β-sheet structures to decrease and have an unstructured order.4
As regards frog meat, parvalbumins are the proteins with the greatest antigenic relevance. They have low MW (around 12 kDa) and are acid, hydrophilic and highly resistant to enzyme degradation. Parvalbumins are found in fish and amphibian muscles, and are considered to be the main allergens of such foods. Hilger et al.34 reported on the implication of a-parvalbumin in a case of anaphylactic shock triggered by the ingestion of thermally-processed frog meat.
Hilger et al.35 conducted another study where they tested the likelihood of cross reactivity between fish and amphibians in codfish-allergic individuals. The blood samples of the researched patients were analyzed by means of in vitro tests. Three out of thirteen samples reacted positively with a-parvalbumin and eleven out of twelve reacted with b-parvalbumin from Rana esculenta. Prick tests were also performed with recombinant parvalbumin in 5 individuals (three were fish-allergic and two were non-allergic). Positive results were obtained for the allergic individuals, attesting the high likelihood of cross-reactivity.
Moreover, figure 3 (lines 1 and 2) shows that low MW proteins remained nearly unaltered when submitted to the treatments. However, proteins whose MW is approximately 56 and 50 kDa were apparently susceptible to cleavage when cooked, if compared to when they were lyophilized or even untreated.
Bernhisel-Broadbent et al.36 investigated salmon and tuna extracts with SDS-PAGE. The result showed a remarkable loss of protein fractions when industrially processed salmon and tuna samples were compared to raw or conventionally cooked extracts. Moreover, the blind placebo-controlled trial (BPCT) confirmed a decrease in allergenicity in two salmon-allergic patients.
The fact that low mW proteins of frog meat are resistant to heat treatment suggests that ingesting cooked, lyophilized or raw frog meat may trigger allergic reactions in genetically predisposed individuals.
The analysis of the beef samples by the same method and submitted to the same treatments has evidenced protein sensitivity to cooking-induced cleavage (line 4) for both high and low molecular weight proteins, while the intermediate proteins remained unaltered. In spite of freeze drying, (line 5) protein bands whose MW is above 116 kDa and have approximately 125, 111 and 108 kDa were observed to be absent, when compared to the in natura sample.
The most important protein in diagnosed beef allergy cases is bovine serum albumin (BSA), whose molecular weight is 66 kDa.37,38 According to Beretta et al.,32 BSA and other serum albumins are also involved in cases of cross reactivity with cow milk.
Sampson39 investigated beef allergy in children with atopic dermatitis, 15.9% of whom tested positive after PT was conducted. However, only 1.8% of the cases were confirmed after BPCT. Werfel et al. 41 obtained positive results for cow's milk allergy in 84% of the children tested through PT, but only 20% of the cases were confirmed by BPCT. Many children with positive PT results for beef are clinically tolerant of several kinds of meat because of enzyme digestion, which can modify the structural features of some food allergens.
Greater resistance to either high or low temperature thermal processing is observed when milk samples are compared to other sources of protein. Several studies have investigated the conformational and linear epitopes that constitute β-lactoglobulin and claim that its tertiary strucuture is probably of crucial importance in the immunoreactivity of the native form of this protein fraction.20,40,41
Host & Samuelson43 investigated allergenic potential of milk in three different preparations: in natura, pasteurized at 75o C for 15 seconds and pasteurized and homogenized at 60o C (175 kg/cm2). PT and BPCT were positive in all the children tested, who were highly prone to allergenicity even for thermally processed samples.
The findings of this experiment corroborate epidemiological data on food allergy worldwide, since cow's milk allergy is much more prevalent than beef allergy in the world's population.19,39,40,43,44
Conclusions
Heat treatment was found to be an efficient denaturant because it fosters the cleavage of proteins from food sources and can often reduce their allergenic potential. Another finding is that some proteins are more resistant to denaturing than others. Cow's milk proteins, for example, are less susceptible to thermal processing. Although frog meat ranked in between milk and beef as regards the thermal resistance of its constituent proteins, there is still much controversy in the literature as to whether or not it can be safely eaten by allergic patients. As a source of protein, beef showed the most sensitivity to the different thermal treatments applied, and hence appears to have low allergenicity. The comsumption of other kinds of meat by genetically predisposed individuals has to be carefully handled and evaluated on an individual basis because no meat or milk can be considered hypoallergenic, and that cross reactivity among sources of protein poses a serious nutritional problem to children with food allergy, especially multi-allergenic ones.
References
1. Ordonez JA. Tecnologia de alimentos: componentes dos alimentos e processos. 1a ed. Artmed. Porto Alegre. 2005. [ Links ]
2. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol 1996; 14 (20): 1269-73. [ Links ]
3. Rance F, Kanny G, Dutau G, Moneret-Valtrin DA. Food hypersensitivity in children: clinical aspects and distribution of allergens. Pediatr Allergy Immunol 1990; 10: 33-8. [ Links ]
4. Davis PJ, Smales CM, James DC. How can thermal processing modify the antigenicity of proteins? Allergy 2001; 56 (67): 56-60. [ Links ]
5. Untersmayr E, Poulsen LK, Platzer MH, Pedersen MH, Boltz-Nitulescu G, Skov PS, Jensen-Jarolim E. The effects of gastric digestion on codfish allergenicity. J. Allergy Clin Immunol 2005; 115 (2): 377-82. [ Links ]
6. Battaisa F, Courcouxb F, Popineaua Y, Kannyc G, Moneret-Vautrinc DA, Denery-Papinia S. Food allergy to wheat: differences in immunoglobulin E-binding proteins as a function of age or symptoms. J Cereal Sc 2005; 42 (1): 109-17. [ Links ]
7. Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, Hirota Y, Oda, H. Soy, isoflavones and prevalence of allergic rhinitis in Japanese women: The Osaka Maternal and Child Health Study. J Allergy Clin Immunol 2005; 115 (6): 1176-83. [ Links ]
8. Host A, Samuelsson EG. Allergic reactions to raw, pasteurized and homogenized/pasteurized cow milk: a comparison. Allergy 1988; 43: 113-7. [ Links ]
9. Restani P, Ballabio C, Cattaneo A, Isoardi P, Terracciano L, Fiocchi A. Characterization of bovine serum albumin epitopes and their role in allergic reactions. Allergy 2004; 59 (78): 21-4. [ Links ]
10. Brenna O, Pompei C, Ortolani C, Pravettoni V, Farioli L, Pastorello E. Technological processes to decrease the allergenicity of peach juice and nectar. J Agric Food Chem 2000; 48: 493-7. [ Links ]
11. Fiocchi A, Restani P, Riva E. Beef allergy in children. Nutrition 2000; 16: 454-7. [ Links ]
12. Nowak-Wegrzyn A. Future approaches to food allergy. Pediatrics 2003; 111 (06): 1672-80. [ Links ]
13. Fiocchi A, Restani P, Riva E. Heat treatment modifies the allergenicity of beef and bovine serum albumin. Allergy 1998; 53: 798-802. [ Links ]
14. Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol 2005; 115: 3-12. [ Links ]
15. Tattini VJ, Parra DF, Pitombo RNM. Influência da taxa de congelamento no comportamento físico-químico e estrutural durante a liofilização da albumina bovina. Brazilian J Pharm Sci 2006; 42 (1): 127-36. [ Links ]
16. Chen T, Oakley DM. Thermal analysis of proteins of pharmaceutical interest. Thermochim Acta 1995; 248: 229-44. [ Links ]
17. Sabrá A, Del Castilho R, Sabrá S, Madi K. Tratamento da Alergia Alimentar. In: Sabrá A, Del Castilho R, Sabrá S, Madi K. Temas de Pediatria (pp. 46-51). Nestle. São Paulo. 1995. [ Links ]
18. Martins ER. Alergia Alimentar. In: Rios JBM, Carvalho LP. Alergia Clínica - Diagnóstico e Tratamento. 1a ed. (pp. 505-23). Revinter. São Paulo. 1995. [ Links ]
19. Szabó I, Eigenmann PA. Allergenicity of major cow's milk and peanut proteins determined by IgE and IgG immunobloting. Allergy 2000; 55: 42-9. [ Links ]
20. Daher S, Tahan S, Sole D, Naspitz CK, Fagundes-Neto U, Morais MB. Cow's milk protein intolerance and chronic constipation in children. Pediatr Allergy Immunol 2001; 12: 339-42. [ Links ]
21. AOAC. Association Official Analytical Chemists. Official Methods of Analysis of the Association Chemist. Washington, DC, USA. 2002. [ Links ]
22. Hsu HW, Vavak DL, Saterlee LD, Miller GA. Multienzyme technique for estimating protein digestibility. J Food Sci 1977; 42 (5): 1262-73. [ Links ]
23. Pires CV, Oliveira MGA, Rosa JC, Cruz GADR, Mendes FQ, Costa NMB. Digestibilidade in vitro e in vivo de Proteínas de Alimentos: Estudo Comparativo. Alim Nutr 2006; 1: 01-09. [ Links ]
24. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680-5. [ Links ]
25. Bannon G, Fu T, Kimber I, Hinton DM. Protein Digestibility and Relevance to allergenicity. Health Perspect 2003; 111 (8): 1122-24. [ Links ]
26. Sathea SK, Teuberb SS, Roux KH. Effects of food processing on the stability of food allergens. Biotechn Advances 2005; 23: 423-9. [ Links ]
27. Fiocchi A, Restani P, Riva E. Meat allergy II - Effects of food processing and enzymatic digestion on the allergenicity of bovine and ovine meats. J Am Coll Nutrition 1995; 14: 245-50. [ Links ]
28. Besler M, Steinhart H, Paschke A. Stability of food allergens and allergenicity of processed foods. J Chromatogr B Biomed Sci Appl 2001; 756: 207-28. [ Links ]
29. Nunes C, Baptista AO. Implicates da reaccáo de Maillard nos alimentos e nos sistemas biológicos. Rev Port Cien Vet 2001; 96 (538): 53-9. [ Links ]
30. Cruz GADR, Oliveira MGA, Pires CV, Gomes MRA, Costa NMB, Moreira MA. Protein quality and in vivo digestibility of different varieties of bean (Phaseolus vulgaris L.). Brazilian J Food Technol 2003; 6 (2): 157-62. [ Links ]
31. Restani P, Restelli AR, Capuano A, Galli CL. Digestibility of technologically treated lamb meat samples evaluated by an in vitro multienzymatic method. J Agr Food Chem 1992; 40: 989-93. [ Links ]
32. Beretta B, Conti A, Fiocchi A. Antigenic determinants of bovine serum albumin. Int Arch Allergy Immunol 2001; 126: 188-95. [ Links ]
33. Roy I, Gupta MN. Freeze-drying of proteins: some emerging concerns. Biotechnol Appl Biochem 2004; 39: 165-77. [ Links ]
34. Hilger C, Grigioni F, Thill L, Mertens L, Hentges F. Severe IgE-mediated anafilaxis following consuption of fried frog legs: definition of a parvalbumin as the allergen in cause. Allergy 2002; 57: 1053-8. [ Links ]
35. Hilger C, Thill L Grigioni F, Lehners C, Falagiani P, Ferrara A, Romano C, Stevens W, Hentges F. IgE antibodies of fish allergic patients cross-react with frog parvalbumin. Allergy 2004; 59: 653-60. [ Links ]
36. Bernhisel-Broadbent J, Strause D, Sampson HA. Fish hypersensitivity: clinical prevalence of altered fish allergenicity caused by various preparation methods. J Allergy Clin Immunol 1992; 90: 622-9. [ Links ]
37. Sampson HA. Update of food allergy. J Allergy Clin Immunol 2004; 113: 805-19. [ Links ]
38. Sampson HA Food allergy: when mucosal immunity goes wrong. J Allergy Clin Immunol 2005; 115: 139-41. [ Links ]
39. Sampson HA. The role of food allergy and mediator release in atopic dermatitis. J Allergy Clin Immunol 1988; 81: 635-9. [ Links ]
40. Werfel S, Cooke SK, Sampson JA. Clinical reactivity to beef in children allergic to cow's milk. J Allergy Clin Immunol 1997; 99: 287-93. [ Links ]
41. Maier I, Okuna VM, Pittner F, Lindner W. Changes in peptic digestibility of bovine p-lactoglobulin as a result of food processing studied by capillary electrophoresis and immunochemical methods. J Chromatogr 2006; 841: 160-7. [ Links ]
42. Host A, Samuelsson EG. Allergic reactions to raw, pasteurized and homogenized/pasteurized cow milk: a comparison. Allergy 1988; 43: 113-7. [ Links ]
43. Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol 1997; 159: 2026-32. [ Links ]
44. Host A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol 2002; 13 (15): 23-8. [ Links ]
![]() Correspondence:
Correspondence:
Tatiana Coura Oliveira.
Fundação Comunitária de Ensino Superior de Itabira.
Rua Venâncio Auguto Gomes, 50 - Prédio Areão - Bairro Major
Lage de Cima.
CEP: 35900-842 - Itabira/MG. Brazil.
E-mail: contato.tatiana@gmail.com
Recibido: 1-VI-2012.
Aceptado: 11-IX-2012.