Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.28 no.3 Madrid Mai./Jun. 2013
https://dx.doi.org/10.3305/nh.2013.28.3.6363
ORIGINAL
Low-fat dairy products consumption is associated with lower triglyceride concentrations in a Spanish hypertriglyceridemic cohort
Asociación del consumo de lácteos desnatados con menores niveles de triglicéridos en una cohorte española de sujetos con hipertrigliceridemia
Jordi Merino1, Rocío Mateo-Gallego2, Nuria Plana1, Ana María Bea2, Juan Ascaso3, Carlos Lahoz4, José Luis Aranda5; On behalf of the Hypertriglyceridemic Registry of the Spanish Arteriosclerosis Society**
1Unitat de Medicina Vascular i Metabolisme. Unitat de Recerca de Lipids i Arteriosclerosi. Departament de Medicina Interna. Hospital Universitari Sant Joan. IISPV. Universitat Rovira i Virgili, y CIBERDEM. Reus. Tarragona. Spain.
2Unidad de Lipidos. Hospital Universitario Miguel Servet. Instituto Aragonés de Ciencias de la Salud, Zaragoza. Spain.
3Servicio de Endocrinología y Nutrición. Departamento de Medicina. Hospital Clínico Universitario de Valencia. Universidad de Valencia. Valencia. Spain.
4Unidad de Arteriosclerosis. Hospital Carlos III. Madrid. Spain.
5Servicio de Medicina Interna. Hospital 12 de Octubre. Madrid. Spain.
**Hypertriglyceridemia Registry of the Spanish Arteriosclerosis Society:, Almagro Múgica F, Álvarez-Sala Walther LA, Argimón Pallas J, Becerra Fernández A, Brea Hernando A, Borrallo Almansa RM, Carrasco Miras F, Civeira Murillo F, Eloy Moreno Bandera FJ, Fabiani Romero F, Fahades Enrich A, Fernández-Miranda Parra C, Ferrando Vela J, Fuentes Jiménez FJ, Galiana López Del Pulgar J, García Arias C, Godoy Rocati D, González Santos P , Gordo Fraile P, Hernández Anguera JM, Hernández Mijares A, Irigoyen Cucalon L, Jansen Chaparro S, Jarauta Simón E, Jiménez Morales JL, Laguna F, López Chozas JM, Martínez Hervas S, Mari Solivellas B, Martis Sueiro A, Masana Marín L, Mediavilla García JD, Morales Coca C, Morillas Ariño C, Mosquera Lozano D, Mostaza Prieto J, Panisello Royo J, Pérez De Juan Romero M, Pérez Silvestre J, Pia Iglesias G, Recarte Andrade C, Ros Rahola E, Ruiz García A, Saenz Aranzubia P, Sánchez Muñoz Torrero JF, Sanclemente Anso C, Sarasa Corral I, Sevilla Moya JC, Sola Izquierdo E, Suarez Tembra M, Toro Santos JM, Trias Vilaguta F, Valdivielso Felices P, Vives Almandoz A, Pinto Sala X, Zambón Rados D.
This work was supported by grants from Ferrer's Group Cardiovascular Area wich had no role in any of the following: the study design; the collection, analysis, and the interpretion of data; the writing of the manuscript; and the decision to submit the manuscript for publication.
ABSTRACT
Introduction: The first line of treatment for hypertriglyceridemic (HTG) includes a well-balanced diet, although the association of dietary components with triglyceride (TG) concentrations in hypertriglyceridemic patients is not fully understood.
Objective: To describe the main dietary patterns in a cohort of hypertriglyceridaemic patients and to evaluate the association between dietary components and TG levels.
Methods: This multicentre cross-sectional study included subjects (n = 1.394) with HTG (TG > 2.25 mmol/L) visiting lipid units affiliated with the Spanish Atherosclerosis Society. A validated 14-item food questionnaire was performed to assess diet. Clinical, anthropometry and biochemical parameters were also obtained.
Results: Two dietary patterns were defined a posteriori by cluster analysis. Patients following the "prudent dietary pattern" (predominantly fish, fruits, vegetables, low-fat dairy and legumes) had lower TG levels than those with the "western dietary pattern" (predominantly red and processed meat products, alcohol, cakes and pastries and sugar) (3.51 ± 2.41 vs. 3.96 ± 3.61 mmol/L, P = 0.002). In a multivariant test, low-fat dairy products (B: -0.089; 95% IC: -16.1, -3.1, P = 0.004) and alcohol intake (B: 0.070; 95% IC: 1.1, 13.1, P = 0.022) were significantly associated with TG concentrations independently of potential confounders.
Conclusions: Mediterranean dietary pattern including low-fat dairy products and abstaining from alcohol intake is highly associated with lower TG concentration in hypertriglyceridaemic patients even under lipid-lowering treatment. The reinforcement in nutritional counselling mainly in these food groups should be done and further specifically studies about the direct association of these and other dietary groups should be carried out to the development of more effective nutritional recommendations.
Key words: Low-fat dairy products. Triglycerides. Hypertriglyceridemic. Dietary patterns. Alcohol.
RESUMEN
Introducción: Una dieta cardiosaludable constituye el tratamiento de primera línea en la hipertrigliceridemia (HTG) aunque la asociación de los diferentes componentes de ésta con la concentración de triglicéridos (TG) en pacientes con HTG no está completamente establecida.
Objetivo: Estudiar los patrones dietéticos en una cohorte de pacientes hipertrigliceridémicos y evaluar la asociación entres los diferentes componentes de la dieta y la concentración de TG.
Métodos: El estudio, multicéntrico y transversal, incluyó sujetos (n = 1.394) diagnosticados de HTG (TG > 2,25 mmol/L) que fueron remitidos a diferentes Unidades de Lípidos pertenecientes a la Sociedad Española de Arteriosclerosis. Se realizó una valoración dietética mediante un cuestionario validado de 14 items además de la obtención de otras variables clínicas, antropométricas y bioquímicas.
Resultados: Se definieron dos patrones dietéticos mediante análisis tipo cluster. Aquellos pacientes que seguían una "dieta cardiosaludable" (en la que predominaba el consumo de pescado, fruta, verduras, lácteos desnatados y legumbres) presentaron menores niveles de TG que aquellos que mantenían una "dieta no cardiosaludable" (con consumo de carne roja, productos cárnicos procesados, alcohol, bollería y azúcar predominantemente) (3,51 ± 2,41 vs. 3,96 ± 3,61 mmol/L, P = 0,002). El análisis multivaríante determinó que el consumo de lácteos desnatados (B: -0,089; 95% IC: -16,1, -3,1, P = 0,004) y el de alcohol (B: 0,070; 95% IC: 1,1, 13,1, P = 0,022) se asoció significativa, e independientemente de otros factores de confusión, con la concentración de TG.
Conclusiones: Un patrón dietético típicamente mediterráneo incluyendo lácteos desnatados y un bajo consumo de alcohol se relaciona con menores concentraciones de TG en pacientes hipertrigliceridémicos, incluso en aquellos con tratamiento hipolipemiante. Queda patente así la importancia y necesidad del refuerzo del consejo dietético en esta población además de futuros estudios que analicen directamente la asociación de éstos y otros grupos de alimentos que permitan el desarrollo y la consecución de recomendaciones nutricionales más efectivas.
Palabras clave: Lácteos desnatados. Triglicéridos. Hipertrigliceridemia. Patrones dietéticos. Alcohol.
Abbreviations
BMI: Body mass index.
DM2: Type 2 diabetes mellitus.
GGT: Gamma-glutamyl transpeptidase.
GIP: Glucose-dependent insulinotrophic polypeptide.
GLP-1: Glucagon-like peptide-1.
GOT: Alanine aminotransferase.
GPT: Aspartate transaminase.
HDLc: High density lipoprotein cholesterol.
HTG: Hypertriglyceridemic.
MS: Metabolic syndrome
TG: Tryglicerides.
Introduction
The association between elevated triglycerides (TG) levels and cardiovascular disease has been widely established.1-3 Hypertriglyceridemic (HTG) is a common lipid metabolism disorder with a high prevalence in the Spanish population (at least 8% in middle-aged male workers and 31% in primary care outpatients).4,5 HTG is a multifactorial disease with an important interaction between genetic and environmental factors, especially with overweight, obesity, peripheral insulin resistance and metabolic syndrome (MS).6-8 The first line treatment for HTG includes the promotion of a healthy lifestyle including daily physical activity, normal body weight and a well-balanced diet.9,10 It is known that certain macronutrients and food groups, such as carbohydrates, fat or alcohol, increase TG concentration, however, the potential impact of the dietary patterns and the association between different dietary components in subjects with HTG is unknown.11-13
The Spanish Atherosclerosis Society created in 2007 the Spanish Hypertriglyceridemia Registry including data from affiliated lipid units throughout Spain.
Therefore, the objective of the present study was to describe the main dietary patterns of this group of patients and to evaluate the possible association between dietary components and TG levels.
Methods
Study design
A detailed description of the study has been previously published.14,15 Briefly, the study included cross-sectional data from the Spanish Hypertriglyceridemia Registry of the Spanish Atherosclerosis Society where patients aged > 18 years of both genders referred to the lipid units belonging to this Society for the screening and treatment of HTG were included. HTG was defined as plasma levels of TG > 2.25 mmol/L in the first fasting blood sample tested in the lipid unit. HTG aetiology was defined by medical criteria and it was classified in: primary (hyperchylomicronaemia, familial combined hyperlipidaemia, familial hypertriglyceridaemia and dysbetalipoproteinaemia), and secondary (to alcohol, MS, type 2 diabetes (DM2) or other causes). The study was approved by the Ethics Committee of the Carlos III Hospital (Madrid, Spain) and by the other participant hospitals in the study.
Lifestyle parameters
Patients were asked about tobacco consumption, physical activity and a dietary assessment was performed. Physical activity evaluation included work activity and leisure-time activity questions with a score from 0 to 4, being 0 the most sedentary level. The short food questionnaire was developed by the Spanish Hypertriglyceridemia Registry and included 14 questions (yes/no) about the frequency of the main foods consumption based on Mediterranean dietary pattern. The food questionnaire was validated in 63 consecutive patients attending to the lipids units of Reus and Zaragoza comparing to the validated 134-items food frequency questionnaire used in PREDIMED trial and a high concordance was observed between both methods.16 All items showed a kappa index upper than 0.75 with a mean concordance index of 0.722. The strongest concordance was observed in added sugar variable with a kappa of 0.876. Daily intake of low-fat dairy showed a concordance of 0.747 between data included in both registers. Alcohol intake was separately registered including the total intake and the kind of beverage consumed during a week.
Clinical and laboratory determinations
Demographic, medical treatment and personal and family background data (coronary disease, cerebrovascular disease, peripheral artery disease, hypertension, diabetes and smoking habits) were registered. Clinical and anthropometric data involved weight, height, calculated body mass index (BMI), waist circumference and blood pressure. The presence of metabolic syndrome was registered, according to The National Cholesterol Education Program (Adult Treatment Panel III) criteria.9,17 Laboratory analyses were locally performed in the lipid units in accordance with standardized methods. Analyses of total cholesterol, TG, high density lipoprotein cholesterol (HDLc), glycaemia, alanine aminotransferase (GOT), aspartate transaminase (GPT), gamma-glutamyl transpeptidase (GGT) were performed using enzymatic and turbidimetric assays. When plasma TG concentration was > 4.52 mmol/L, LDLc was calculated by using the Friedewald formula.
Statistical analysis
Normality distribution of variables was assessed with the Kolmogorov-Smirnov test. Differences in anthropometrical or biochemical data were analyzed using the Kruskall-Wallis test or chi-squared test when indicated. To identify major baseline dietary patterns and to segregate subjects based on the similar diets we used a two-step cluster analysis with Schwarz Bayesian criteria. Unadjusted and adjusted lineal stepwise logistic regression models were performed to determinate predictors of TG concentrations in all of the participants including TG levels as dependent variable and the 14 items of the food questionnaire plus alcohol intake as independent ones. Adjusted factors were age, gender, BMI, presence of type 2 diabetes mellitus, HTG aetiology, lipid-lowering drugs, physical activity level and tobacco consumption. P-values were calculated as two-sided; a p-value of less than 0.05 was considered statistically significant. SPSS version 18.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
Dietary patterns
Participants were classified a posteriori into two major dietary patterns according to 14 items data using a cluster analysis. The cluster labelled "prudent dietary pattern" included daily intake of fruits, vegetables and low-fat dairy products, three or more servings a week of fish and two o more servings a week of legumes (n = 682). The cluster labelled "western dietary pattern" included daily intake of sugar, daily alcohol, consumption of red meat and processed meat products (more than two servings a week of each one) and cakes, pastries and other in baked goods with added sugar more than once a week, (n = 557). Daily consumption of olive oil and salt were closely related in the two dietary patterns. Furthermore, consumption of eggs (more than 3 units a week), nuts (two or more servings a week) and crisps or other snacks more than once a week were excluded from both dietary patterns. Loading factors of food across these major food patterns are presented in table I.
Clinical and biochemical characteristics between dietary patterns
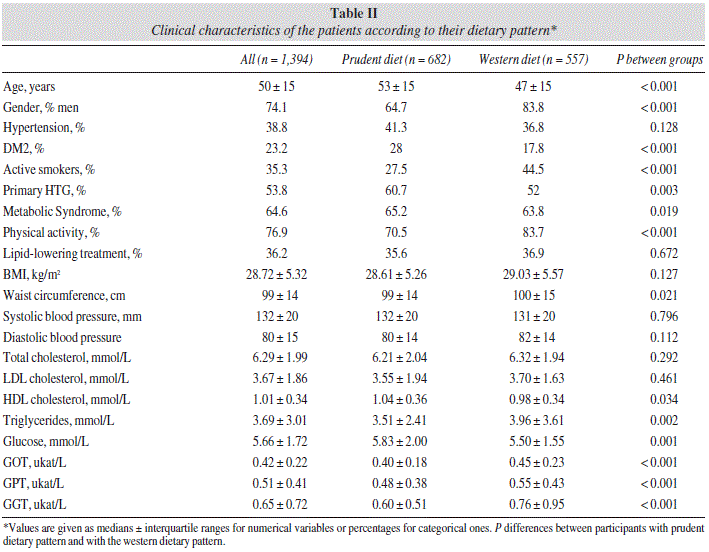
Anthropometric, demographic and biochemical differences according to dietary patterns (prudent dietary pattern and western dietary pattern) are shown in table II. Those subjects following a prudent dietary pattern had significantly lower TG levels compared to those with a western dietary pattern subjects (3.51 ± 2.41 vs. 3.96 ± 3.61 mmol/L, P = 0.002). HDLc was higher in participants with the prudent dietary pattern (1.04 ± 0.36 vs. 0.98 ± 0.34 mmol/L, P = 0.034) as well as glycaemia (5.83 ± 2.00 vs. 5.50 ± 1.55 mmol/L, P < 0.001). In the prudent dietary pattern study group, there were more patients with DM2 (28.0 vs. 17.8%, P < 0.001), primary HTG (60.7 vs. 52.0%, P = 0.003) and MS (65.2 vs. 63.8%, P = 0.019) than in the western dietary pattern group. Moreover, the percentage of active smokers (44.5 vs. 27.5%, P < 0.001) and sedentary lifestyle (83.7 vs. 70.5%, P < 0.001) were higher in individuals following the western dietary pattern compared with those patients with the prudent dietary pattern. When patients with DM2 (n = 340) were excluded from the analysis, differences in glycaemia between participants in the prudent dietary pattern compared to participants in the western dietary pattern (5.38 ± 0.94 vs. 5.33 ± 0.94 mmol/L, P = 0.385) were not observed and TG concentration remained significantly lower for individuals with the prudent dietary pattern (3.53 ± 2.31 vs. 3.79 ± 3.59 mmol/L, P = 0.041).
Triglyceride concentrations determinants
The multiple linear stepwise regression model was performed to assess the relation between food patterns and TG concentration. Unadjusted analysis showed significant associations between daily consumption of low-fat dairy (B: -0.092; 95% CI: -16.61, -3.95, P= 0.001) and alcohol intake (B: 0.084; 95%CI: 2.98, 15.31, P = 0.004) with TG levels. After adjusting the analysis by age, gender, BMI, DM2, lipid-lowering drugs and physical activity level, the significance remained between low-fat dairy (B: -0.089; 95%CI: -16.1, -3.1, P = 0.004) and alcohol intake (B: 0.070. IC: 95% 1.1, 13.1, P = 0.022) (fig. 1).
Figure 2 shows the differences in TG according to each dietary pattern and the consumption of low-fat dairy products. Those patients in the western dietary pattern group with regular intake of low-fat dairy (n = 231) had lower TG concentration than individuals in the same group but without habitual consumption of low-fat dairy (n = 326) (3.65 ± 2.89 vs. 4.17 ± 4.11 mmol/L, P = 0.033). The same results were observed in individuals in the prudent dietary pattern group; non-habitual consumers of low-fat dairy (n = 149) had higher TG levels than habitual consumers of low-fat dairy (n = 533), (3.61 ± 3.57 vs. 3.50 ± 2.14 mmol/L, P = 0.015).
Discussion
This study was firstly aimed to determine the main dietary patterns in a group of hypertriglyceridemic patients included in a Spanish Hypertriglyceridaemic Registry. Secondly, we aimed to evaluate the association between dietary components and TG concentrations in these patients. We mainly observed that a dietary pattern characterized by: daily consumption of fruits, vegetables and low-fat dairy products, three or more servings a week of fish and two or more servings a week of legumes was associated with lower TG concentrations. This pattern also included the lack of consumption of red and processed meat products more than two servings a week, daily intake of sugar and alcohol, and more than once a week of cakes, pastries and other in baked goods with added sugar.
Low-fat dairy products and alcohol intake were the main food groups related to lower TG levels in our study population. According with this observation, different studies have suggested a beneficial role of low-fat dairy consumption on MS and DM2, but not yet in TG concentrations.18-21 It is complex to define the physiologic effect of low-fat dairy products on TG concentrations, but several studies suggested that the insulinotropic effect of milk has been attributed to casein and other soluble whey proteins that increased the incretin hormones concentrations.22-25 It seems that the mechanism by which whey proteins induce hyperinsulinaemia involve two separate pathways: one connected to the significant increment in certain amino acids, such as branched chain amino acids; and other one connected through incretins, with glucose-dependent insulinotrophic polypeptide (GIP) being particularly stimulated.26 It was reported that saturated fatty acids decrease postprandial biodisponibility of glucagon-like peptide-1 (GLP-1) and GIP suggesting that the benefit of low-fat dairy products in insulin-mediated metabolic pathways are not attributable in whole dairy products.24,27
The effect of alcohol in TG levels has been widely proved and our data provide more evidence on the matter in a specific well characterized cohort of hypertriglyceridemic patients with a relative regression coefficient of alcohol of 7%.13,28,29 A meta-analysis including 42 studies the authors described that TG concentrations increased by 0.19 mg/dl per gram of alcohol consumed per day and 5.69 mg/dl (2.49 to 8.89) per 30 g consumed a day, representing a 5.9% increase over baseline30.
Olive oil and nuts are representative foods of the traditional Mediterranean dietary pattern, and their properties are associated with a lower risk of MS and cardiovascular disease.31-33 However, we did not observe a relationship between these foods and the prudent dietary pattern. It may be explained by the fact that the majority of participants in the study (97.1% in the prudent dietary pattern group and 84.4% in the western dietary pattern group) consumed olive oil every day and did not present a consumption of nuts two times per week (18.9% in prudent dietary pattern group and 15.8% in western dietary pattern group), thus this was not considered a discriminating factor for the different dietary patterns.
Another observation of this work is the larger number of subjects with MS, DM2 and primary HTG found in the group of the prudent dietary pattern. It might be explained by the possibility that these patients had probably received previous nutritional advice from a general practitioner before being referred to the lipids unit. Other explication of this observation is the reverse cause of cross-sectional studies, particularity in studies focused on lifestyle and cardiovascular risk factors.
One limitation of our study is its observational nature; therefore, we cannot conclude that increase in low-fat dairy intake reduces TG concentration in causal manner. We included in this study Spanish patients, therefore the extrapolation regarding the association of dietary patterns and foods with TG must be performed with caution in other study populations. Another inherent limitation is related to the potential measurement error in the dietary assessment by using a short food questionnaire which provides subjective information.
Conclusions
This is the first study, to our knowledge, carried out in specific hypertriglyceridaemic patients evaluating the main food predictors of TG concentrations. We mainly observed that daily intake of low-fat dairy products are related to lower TG concentrations whereas alcohol consumption was directly associated, both in patients with primary or secondary HTG even if they were under lipid-lowering therapy. A dietary pattern including fruits and vegetables, legumes, fish and low-fat dairy seems to be a better dietary pattern associated with lower TG concentration. Interventional studies in different aethiology of hypertriglyceridemic in hypertriglyceridemic subjects with specific foods are needed to elucidate and clarify the associations found in this study.
Acknowledgments
We especially offer our acknowledgement to J Fernandez-Ballart and R Ferré for the statistical assistance.
Conflict of interests, source of funding and authorship
The authors of the manuscript declare no conflicts of interest. J. Merino, R. Mateo-Gallego (complete CRF and wrote the manuscript) N. Plana, A. M. Bea, J. Ascaso (design the study and enrolled the participants) C Lahoz and J. Merino (performed the statistical analysis) J. L. Ascaso (reviewed the final version). We considered that the manuscript represents valid work, have reviewed the final version of the submitted manuscript, and approve it for publication. No significant amount of data reported in this manuscript has been published elsewhere or is under consideration for publication elsewhere. There are no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter or materials discussed in this manuscript.
References
1. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2011; 123 (20): 2292-333. [ Links ]
2. Jones A. Triglycerides and Cardiovascular Risk. Heart 2012; (Epub ahead of print). [ Links ]
3. Boullart AC, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta 2012; 1821 (5): 867-75. [ Links ]
4. Sánchez-Chaparro MA, Román-García J, Calvo-Bonacho E, Gómez-Larios T, Fernández-Meseguer A, Sáinz-Gutiérrez JC, Cabrera-Sierra M, García-García A, Rueda-Vicente J, Gálvez-Moraleda A, González-Quintela A. Prevalence of cardiovascular risk factors in the Spanish working population. Rev Esp Cardiol 2006; 59 (5):421-30. [ Links ]
5. Laclaustra M, Ordoñez B, Leon M, Andres EM, Cordero A, Pascual-Calleja I, Grima A, Luengo E, Alegria E, Pocovi M, Civeira F, Casasnovas-Lenguas JA. Metabolic syndrome and coronary heart disease among Spanish male workers: a case-control study of MESYAS. Nutr Metab Cardiovasc Dis 2012; 22 (6): 510-6. [ Links ]
6. Carmena R, Ascaso JF, Real JT. Impact of obesity in primary hyperlipidemias. Nutr Metab Cardiovasc Dis 2001; 11 (5): 354-9. [ Links ]
7. Van Der Kallen CJ, Voors-Pette C, De Bruin TW. Abdominal obesity and expression of familial combined hyperlipidemia. Obes Res 2004; 12 (12): 2054-61. [ Links ]
8. Veerkamp MJ, De Graaf J, Stalenhoef AF. Role of insulin resistance in familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol 2005; 25 (5): 1026-31. [ Links ]
9. Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). JAMA 2001; 285 (19): 2486-97. [ Links ]
10. Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28 (6): 1039-49. [ Links ]
11. Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR Jr, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 2010; 92 (4): 954-9. [ Links ]
12. Mozzafarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009; 63 (Suppl. 2): S22-33. [ Links ]
13. Brinton EA. Effects of ethanol intake on lipoproteins and atherosclerosis. Curr Opin Lipidol 2010; 14 (2): 108-14. [ Links ]
14. Ascaso JF, Millán J, Mateo-Gallego R, Ruiz A, Suárez-Tembra M, Borrallo RM, Zambon D, González-Santos P, Peres-deJuan M, Ros E; Hypertriglyceridemia Registry of Spanish Arteriosclerosis Society. Prevalence of metabolic syndrome and cardiovascular disease in a hypertriglyceridemic population. Eur J Intern Med 2010; 22 (2): 177-81. [ Links ]
15. Valdivielso P, Pintó X, Mateo-Gallego R, Masana L, Alvarez-Sala L, Jarauta E, Suárez M, García-Arias C, Plana N, Laguna F; Registro de HTG de la SEA. Clinical features of patients with hypertriglyceridemia referred to lipid units: registry of hypertrigliceridemia of the Spanish Arteriosclerosis Society. Med Clin (Barc) 2011; 136 (6): 231-8. [ Links ]
16. Fernández-Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez-Bauer M, Martínez-González MA, Salas-Salvadó J, Martín-Moreno JM. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 2011; 103 (12): 1808-16. [ Links ]
17. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109 (3): 433-8. [ Links ]
18. Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008; 117 (6): 754-61. [ Links ]
19. Pereira MA, Jacobs DR Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA 2002; 287 (16): 2081-9. [ Links ]
20. Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006; 29 (7): 1579-84. [ Links ]
21. Larsson SC, Virtamo J, Wolk A. Dairy consumption and risk of stroke in Swedish women and men. Stroke 2012; 43 (7): 1775-80. [ Links ]
22. Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004; 80 (5): 1246-53. [ Links ]
23. Frid AH, Nilsson M, Holst JJ, Björck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 2005; 82 (1): 69-75. [ Links ]
24. Esteves de Oliveira FC, Pinheiro Volp AC, Alfenas RC. Impact of different protein sources in the glycemic and insulinemic responses. Nutr Hosp 2011; 26 (4): 669-76. [ Links ]
25. Pal S, Radavelli-Bagatini S. The effects of whey protein on cardiometabolic risk factors. Obes Rev 2012. (Epub ahead of print). [ Links ]
26. Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr 2007; 85 (4): 996-1004. [ Links ]
27. Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 2004; 287 (3): R524-33. [ Links ]
28. Liangpunsakul S. Relationship between alcohol intake and dietary pattern: findings from NHANES III. World J Gastroenterol 2010; 16 (32): 4055-60. [ Links ]
29. Brinton EA. Effects of ethanol intake on lipoproteins. Curr Atheroscler Rep 2012; 14 (2): 108-14. [ Links ]
30. Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ 1999; 319 (7224): 1523-8. [ Links ]
31. López-Miranda J, Pérez-Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI, Escrich E, Ordovás JM, Soriguer F, Abiá R, de la Lastra CA, Battino M, Corella D, Chamorro-Quirós J, Delgado-Lista J, Giugliano D, Esposito K, Estruch R, Fernandez-Real JM, Gaforio JJ, La Vecchia C, Lairon D, López-Segura F, Mata P, Menéndez JA, Muriana FJ, Osada J, Panagiotakos DB, Paniagua JA, Pérez-Martinez P, Perona J, Peinado MA, Pineda-Priego M, Poulsen HE, Quiles JL, Ramírez-Tortosa MC, Ruano J, Serra-Majem L, Solá R, Solanas M, Solfrizzi V, de la Torre-Fornell R, Trichopoulou A, Uceda M, Villalba-Montoro JM, Villar-Ortiz JR, Visioli F, Yiannakouris N. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr Metab Cardiovasc Dis 2010; 20 (4): 284-94. [ Links ]
32. Casas-Agustench P, López-Uriarte P, Bulló M, Ros E, Cabré-Vila JJ, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis 2010; 21 (2): 126-35. [ Links ]
33. Ros E. Olive oil and CVD: accruing evidence of a protective effect. Br J Nutr 2012; 108 (11): 1931-3. [ Links ]
![]() Correspondence:
Correspondence:
Rocío Mateo-Gallego.
Unidad de Lípidos (Medicina Interna).
Hospital Universitario Miguel Servet.
C/ Padre Arrupe, s/n.
50009 Zaragoza. Spain.
E-mail: rmateo.iacs@aragon.es
Recibido: 9-XII-2012.
Aceptado: 29-I-2013.