My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.28 n.4 Madrid Jul./Aug. 2013
https://dx.doi.org/10.3305/nh.2013.28.4.6504
ORIGINAL / OBESIDAD
Body mass index, abdominal obesity, body fat and migraine features in women
Índice de masa corporal, obesidad abdominal, grasa y las características de la migraña en mujeres
Vanessa Rossoni de Oliveira1, Fernanda Camboim Rockett1,2, Kamila Castro1, Alexandre da Silveira Perla3, Màrcia Lorena Fagundes Chaves3,4 and Ingrid D. Schweigert Perry1,4
1Food and Nutrition Research Center. Hospital de Clínicas de Porto Alegre. Universidade Federal do Rio Grande do Sul. Porto Alegre. Brazil
2Post-Graduation Program in Medicine: Medical Sciences. Universidade Federal do Rio Grande do Sul. Brazil
3Neurology Service. Hospital de Clínicas de Porto Alegre. Porto Alegre. Brazil
4Department of Internal Medicine. School of Medicine. Universidade Federal do Rio Grande do Sul. Porto Alegre. Brazil
Sources of financial support: Fundo de Incentivo á Pesquisa e Eventos (FIPE/HCPA), Pró-Reitoria de Pesquisa (PROPESQ-UFRGS), and the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
ABSTRACT
Background: Studies seeking to establish an association between migraine and anthropometric parameters have thus far been inconclusive. Furthermore, drugs used for migraine prophylaxis may be associated with changes in body weight.
Objective: To investigate the potential association of anthropometric parameters and body fat percentage with attack patterns and use of prophylactic medication in migraineurs.
Methods: Cross-sectional study that assessed the body mass index, waist circumference, body fat percentage and related clinical variables (characteristics of attacks and the use of prophylactic medication) in female outpatients with migraine.
Results: 166 female migraineurs aged > 18 years (mean age, 45 ± 14 years) were included in the study. Migraine without aura was most prevalent (71.7%). Mean body mass index and body fat percentage were 27.8±6.0 kg/m2 and 36.4 ± 8.3% respectively. Body mass index and waist circumference were weakly correlated with frequency of attacks over 6 months (rs = 0.162, p < 0.05 and rs = 0.187, p < 0.05 respectively). These correlations remains weak considering only premenopausal women, but disappear in the older women. Stratification of analysis by migraine type field shows a moderate correlation between migraine with aura and frequency of attacks over 6 months and body mass index (rs = 0.369, p < 0.05), as well as waist circumference (rs = 0.423, p < 0.01). Patients who were on prophylactic medication had higher body mass index, waist circumference, and body fat percentage values (p < 0.01, Student t-test).
Conclusion: This study revealed a potential, though tenuous association between migraine and anthropometric parameters and frequency of attacks, which does not reflect on the duration, severity, and disability of attacks, with patterns differing by migraine type, reproductive age and prophylactic medication.
Key words: Migraine. Obesity. Overweight. Abdominal obesity. Body fat.
RESUMEN
Introducción: Los estudios que tratan de establecer una asociación entre la migraña y los parámetros antropométricos hasta ahora han sido poco concluyentes. Además, los fármacos utilizados para la profilaxis de la migraña pueden estar asociados con cambios en el peso corporal.
Objetivos: Investigar la posible asociación de los parámetros antropométricos y el porcentaje de grasa corporal con patrones de ataque y el uso de la profilaxis en los pacientes con migraña.
Métodos: Estudio transversal que evaluó el índice de masa corporal, circunferencia de cintura, porcentaje de grasa corporal y las variables clínicas (características de los ataques y uso de medicación) en mujeres con migraña.
Resultados: 166 mujeres con migraña > 18 años (edad media, 45 ± 14 años) fueron incluidos en el estudio. Migraña sin aura era más frecuente (71,7%). La media del índice de masa corporal y porcentaje de grasa corporal fueron 27,8 ± 6,0 kg/m2 y 36,4 ± 8,3%, respectivamente. Índice de masa corporal y la circunferencia de cintura se correlacionaron débilmente con la frecuencia de los ataques durante 6 meses (rs = 0,162, p < 0,05 y r = 0,187, p < 0,05, respectivamente). Estas correlaciones se mantiene débil considerando sólo las mujeres premenopáusicas, pero desaparecen en las mujeres mayores. La estratificación de los análisis por tipo migraña muestra una correlación moderada entre la migraña con aura y la frecuencia de los ataques de más de 6 meses y el índice de masa corporal (rs = 0,369, p < 0,05), así como la circunferencia de cintura (rs = 0,423, p < 0,01) . Los pacientes que estaban tomando medicamentos profilácticos tuvieron un mayor índice de masa corporal, circunferencia de la cintura, y los valores de porcentaje de grasa corporal (p < 0,01, prueba t de Student).
Conclusiones: Este estudio reveló un potencial, aunque débil asociación entre la migraña y los parámetros antropométricos y la frecuencia de ataques, que no refleja la duración, la gravedad y la incapacidad de los ataques, que tienen diferentes modelos según el tipo de migraña, la edad reproductiva y la medicación profiláctica.
Palabras clave: Migraña. Obesidad. Sobrepeso. Obesidad abdominal. Grasa corporal.
Abbreviations
ABEP: Associação Brasileira de Empresas de Pesquisa /Brazilian Association of Research Companies.
BF%: Body Fat Percentage.
BMI: Body Mass Index.
HCPA: Hospital de Clínicas de Porto Alegre.
MIDAS: Migraine Disability Assessment Test.
MwA: Migraine With Aura.
MwoA: Migraine Without Aura.
WC: Waist circumference.
Introduction
Migraine, one of the main primary headache disorders, is a benign, highly prevalent neurological condition that is most common in the economically productive population,1 Caucasians,2 and women.3 Migraine is characterized by recurring moderate to severe headaches, with a frontotemporal, unilateral or bilateral distribution, pulsating quality, accompanied by nausea, vomiting, photophobia, phonophobia, or osmophobia, occurs in attacks lasting 4 to 72 hours, and may be preceded by focal neurological phenomena known as aura.4,5 Migraine is estimated to affect 34.5% of adult women and 20.1% of adult men in the general population.3 In Brazil, a nationwide study found a migraine prevalence rate of 15.2%.6 In the U.S., the estimated national cost burden of migraine exceeds $11 billion per year.7
Obesity, a chronic, multifactorial disease characterized by an excessive buildup of adipose tissue,8 is a global public health problem.9 Approximately 60% of the adult U.S. population can be classified as overweight or obese,10 the prevalence of obesity and overweight in Brazilian adults is 14.7% and 49% respectively.11 Studies estimate that the annual medical expenditure attributable to obesity in the U.S. is on the order of $147 billion.12
Recent research has suggested that an association exists between obesity and migraine.13 However, epidemiological studies conducted in Brazil6 and else-where14,15 have failed to find any association between body mass index (BMI) and migraine prevalence. Nevertheless, Bigal et al.14,16 found that overweight is associated with greater frequency and severity of migraine attacks. Although clinical and epidemiological trials have demonstrated a relationship between headache and BMI, there is little information on the relationship between body fat distribution -namely, central obesity- or body fat percentage and migraine.17 On the other hand, migraine sufferers may be subject to changes in body weight produced by the use of prophylactic medications.18 Most drugs used in migraine prophylaxis promote weight gain, although some appear to have no influence on body weight and some may even promote weight loss.18,19
This study sought to ascertain whether anthropometric parameters and body fat percentage can influence the pattern of migraine attacks.
Methods
This cross-sectional study used a convenience sampling strategy. We recruited consecutively 166 female migraineurs aged > 18 years, treated at the outpatient Headache Clinic of the Department of Neurology at Hospital de Clínicas de Porto Alegre (HCPA), a tertiary referral hospital in Rio Grande do Sul, Brazil, between March 2010 and August 2011. All had a diagnosis of migraine made by a neurologist (ASP) according to International Headache Society criteria.5
Sociodemographic data (age, marital status, educational level, self-reported skin color, and socioeconomic status), clinical information [(classification of migraine, frequency and duration of attacks, migraine family history, reproductive age (pre and postmenopausal) and medications used)], data on disability, and anthropometric parameters (weight, height, and waist circumference) were collected, by means of a history and physical examination, during an investigator-led patient encounter (a special visit to apply the study protocol). The severity and disability caused by attacks was assessed with a visual analog pain and Migraine Disability Assessment Test - MIDAS criteria.20 Socioeconomic status was defined on the basis of the Brazilian Association of Research Companies (Associagao Brasileira de Empresas de Pesquisa, ABEP) Economic Classification Criterion, which uses purchasing power to stratify the population into five socioeconomic classes, A through E, with A representing the richest stratum of society and E, the poorest21. Weight and height were used to calculate BMI, using the formula BMI = (weight [kg]/height [m]2), and nutritional status classification was based on the World Health Organization cutoff points for adults22 and the Lipschitz cutoffs for the elderly.23 Waist circumference (WC) was measured at the narrowest point of the trunk and classified according to World Health Organization standards,24 with WC > 80 or > 88 cm representing increased or substantially increased risk of cardiovascular disease and metabolic complications respectively. Body fat percentage (BF%) was measured by the bioelectrical impedance method with a Maltron body composition analyzer (BF-906, Maltron International Ltd, Essex, UK).
This study was approved by the Hospital de Clínicas de Porto Alegre Research Ethics Committee with protocol #09-523. All participants provided written informed consent.
Categorical variables were expressed as absolute and relative frequencies, and continuous variables, as mean and standard deviation or median and interquartile range as appropriate. The chi-square test was used to test for association between categorical variables; the Student t-test, for comparison of means; and Spearman's rank correlation coefficient to test for correlations between anthropometric parameters and frequency, severity, and duration of migraine attacks. Data were analyzed in the Statistical Package for the Social Sciences 18.0 software, and results were considered significant when p < 0.05.
Results
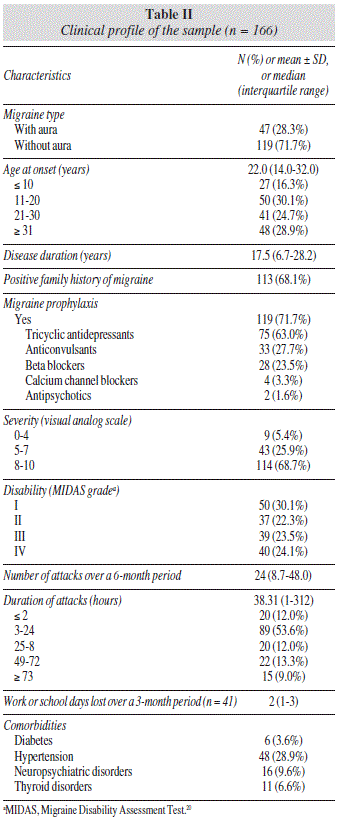
The sociodemographic profile of the sample is described in table I. There were no associations between socioeconomic and educational level, marital status, or age and migraine severity as measured both by the MIDAS instrument and a visual analog pain scale. Age did not correlate with attack frequency or duration either. Seventy-three (44.0%) were employed at the time of the study; of these, 41 (56.1%) reported absenteeism due to migraine attacks (table II).
Migraine without aura was highly prevalent. Age at onset of first migraine attack was < 20 years, and the median time elapsed since the first migraine was 17.5 years (interquartile range, 6.7-28.2 years). Substantial number of patients reported a family history of migraine. Migraine-induced disability, as measured by the MIDAS instrument ranged from grade I to grade IV. On average, patients had experienced 24 migraine attacks over the 6 months preceding the study, with the predominant duration of attacks being < 24 hours. Most patients reported a mean attack severity of 8 to 10 on a visual analog pain scale, and most were on prophylactic medications. Hypertension was the main comorbidity in this sample (table II).
According to BMI and WC values, a substantial number of patients were overweight and thus at risk of cardiovascular disease and metabolic complications (table III). There were no statistically significant differences in BMI, WC, or BF% between the MwA and MwoA groups.
Although the associations were weak, BMI and WC correlated positively with the number of migraine attacks over a 6-month period (table IV), whereas BF% did not.
Considering only premenopausal women, there was also a weak correlation between BMI and WC and the number of crises over a 6-month period. However, in the postmenopausal women this correlation disappear (table IV).
Even if there was no difference in anthropometric parameters between types of migraine (table III), in migraine with aura, there was a moderate, positive correlation between BMI and WC and attack frequency over 6 months, whereas neither parameter was associated with attack frequency in patients who experienced migraine without aura (table IV).
Analysis of attack duration and severity (as measured on a visual analog scale) in the pooled sample (migraine with and without aura) revealed no correlation with anthropometric parameters (table IV). If considered women in pre and post menopausal age there was also no correlation. However, in the pooled sample there was a positive correlation between attack severity and duration (rs = 0.229; p = 0.003).
The degree of migraine-related disability, as measured with the MIDAS instrument, was not associated with BMI, WC, or BF%, even in the total sample, even if considered the reproductive age (chi-squared test).
There was no association between the class of prophylactic medication used and BMI, WC, or BF%, the exception of tricyclic antidepressants, which were associated with increased WC (p = 0.044). As compared to patients who were not on pharmacological prophylaxis, patients who were receiving any prophylactic medication had a greater BMI (28.6 ± 6.0 kg/m2 vs. 25.6 ± 5.6 kg/m2, p = 0.003), BF% (37.6 ± 7.6% vs. 32.1 ± 8.6%, p < 0.001), and WC (86.4 ± 12.8 cm vs. 79.1 ± 12.4, p = 0.001) (Student t-test).
Discussion
As reported elsewhere in the literature,6,25 migraine without aura was predominant in our sample.5 The greater frequency of migraine among patients in socioeconomic classes B and C, as well as the high representativeness of participants self-reporting as white, as described previously,26 may be due to the nature of the sample, which consisted of outpatients treated at a public health care in Southern Brazil. This restriction may limit the interpretation of the influence of these factors on attack severity and migraine-related disability in the study population. The reported pain severity and high rate of absenteeism among employed participants27 also reflect the reality of an outpatient sample.
Approximately 60% of participants in the study sample were overweight or obese. This is a worrisome finding, which corroborates the global epidemic status of obesity.9 Considering overweight alone, the findings of this study are similar to those of previous population surveys of migraineurs.6,14,16 However, our data suggest a greater prevalence of overweight than a previous, nationwide population study, which found that less than 50% of people with migraine were overweight or obese.6 Whereas Queiroz et al.6 reported an obesity rate similar to that of the overall Brazilian population,11 our sample had a higher prevalence of obesity (roughly 12% vs. 26.5% respectively). These aspects should be considered in the context of the sample of the present study, which, again, consisted of outpatients whose characteristics may have been distinct from those of population study samples, both in terms of the use of prophylactic medication and in terms of regional differences in the prevalence of obesity.11
Although Peterlin et al.17 found that migraine was more prevalent in obese individuals, there is no clear relationship between BMI and migraine, as demonstrated by the fact that other population studies failed to find similar results.6,15,16 However, an association between BMI and frequency and severity of migraine attacks has been reported by some authors,13,16 and appears to have been corroborated in our sample, with a potential correlation, however weak, between greater frequency of attacks and higher BMI. After stratification of analysis by migraine type, this correlation was found to be stronger in the migraine with aura group, as was the correlation between central distribution of body fat and frequency of attacks. These data are consistent with those of a previous study of severely obese female migraineurs, in which most participants had a diagnosis of migraine with aura.28 The authors of that study suggested that the presence of aura may be associated with high estrogen levels, and that extraovarian production of estrogen in adipose tissue might account for this phenomenon, which does not occur in migraine without aura, where attacks are associated with low levels of estrogen.
A large population study found that central distribution of body fat was associated with increased prevalence of migraine in women, although the correlation did not hold after the age of 55.17 Contradicting the aforementioned findings of Horev et al.,28 who only examined severely obese women (with a potentially greater extraovarian output of estrogen) of reproductive age, Peterlin et al.17 found that the association between WC and migraine ceased to be significant in less severely obese women over the age of 55, with a presumably lower estrogen output. According to Bond et al.,29 there appears to be a relationship between obesity and migraine, particularly in reproductive-aged women.
Although in our study, we found a correlation between BMI and WC and higher frequency of crises, this was a weak correlation that remained weak, even when the sample was stratified into women in pre and post menopausal. We must consider that our sample consisted predominantly of pre menopausal women. Although with limited sample, the correlation is lost in the post menopausal women, which may be contributing to that proposed by the literature that points to associated endocrine effects.17,29.
Besides the fact that in this study, approximately 37.3% of participants were aged > 50 years, most were moderately obese (overweight or grade 1 obesity), and 57.8% had a WC above normality cutoffs. All these aspects may have contributed to the weakness of the detected correlations. These aspects could also be associated with the lack of correlation between anthropometric parameters and migraine-related disability. Bigal et al.16 found that disability varied according to BMI, with some disability occurring in 32% of patients with a normal BMI, 37.2% of overweight patients, 38.4% of obese patients, and 40.9% of severely obese ones. Bond et al.30 reported that severely obese migraineurs experienced improvement in symptoms after weight loss due to bariatric surgery.
Possible relationships between migraine and obesity have also been partly explained by the presence of inflammatory mediators common to both conditions.30 Evidence shows that an improved understanding of the role of adipose tissue in activation of the inflammatory cascade could lead to suggestions of new treatment and prevention strategies for the reduction of obesity-associated morbidity and mortality. This, in turn, could be an important breakthrough for migraine, which is associated with neurovascular inflammation.31-33 Furthermore, migraine and obesity alike are comorbidities associated with a variety of cardiovascular diseases and are a risk factor for stroke, particularly in women who suffer from migraine with aura.34
Of the various medications available for migraine prophylaxis, those most often used in the study population were tricyclic antidepressants (particularly amitriptyline), anticonvulsants (valproic acid and topiramate), and beta blockers (propranolol). Amitriptyline and valproic acid are associated with the most severe weight gain,35 whereas propranolol was associated with less weight gain in some studies.36,37 Conversely, topiramate has been reported to aid weight loss.37,38 In our sample, BMI, BF%, and WC values were higher among patients on migraine prophylaxis, which might indicate an association between prophylactic medication and changes in body weight. When analysis was stratified by drug class, the only detectable association was between use of tricyclics and WC; the relationship between use of these medications and BMI, as previously reported in the literature,36,39 was not present in our sample. One limitation to the interpretation of these findings was the unavailability of anthropometric data for the participants as of the start of prophylactic therapy.
In conclusion, this study demonstrated a potential, though tenuous, connection between migraine and certain anthropometric parameters (BMI and WC) with respect to the frequency of migraine attacks, with no reflection, however, on their duration or severity. Reproductive age and migraine type appears to be a determinant of this correlation, which was stronger in migraine with aura and disappear in post-menopausal women. These results suggest that migraine prophylaxis strategies should take into account not only pharmacological aspects, but also management of nutritional status.
References
1. Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin 2009; 27: 321-34. [ Links ]
2. Stewart WF, Lipton RB, Liberman J. Variation in migraine prevalence by race. Neurology 1996; 47 (1): 52-9. [ Links ]
3. Bigal M, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology 2006; 67: 246-51. [ Links ]
4. Krymchantowski AV, Moreira Filho PF. Atualização no tratamento profilático das enxaquecas. Arq Neuro-Psiquiatr 1999; 57: 513-9. [ Links ]
5. IHS. International Headache Society. The International Classification of Headache Disorders - Part One - The primary headaches. Cephalalgia 2004; 24 (Suppl. 1): 23-136. [ Links ]
6. Queiroz LP, Peres MFP, Piovesan EJ, Kowacs F, Ciciarelli MC, Souza JA, et al. A nationwide population-based study of migraine in Brazil. Cephalalgia 2009; 29: 642-9. [ Links ]
7. Hawkins K, Wang S, Rupnow M . Direct cost burden among insured US employees with migraine. Headache 2008; 48: 553-63. [ Links ]
8. Fandiño J, Benchimol AK, Coutinho WF, Appolinário JC. Cirurgia bariátrica: aspectos clínico-cirúrgicos e psiquiátricos. R Psiquiatr RS 2004; 26: 47-51. [ Links ]
9. WHO-World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894) (document on the Internet). 2000 (cited 2013 Feb 07). Available from: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/index.html. [ Links ]
10. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity in among US adults, 1999-2008. JAMA 2010; 303: 235-41. [ Links ]
11. Brasil. Ministério da Saúde, Ministério do Planejamento, Orçamento e Gestão. Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2008-2009 (document on the Internet). 2011 (cited 2013 Feb 07). Available from: http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_2009_encaa/pof_20082009_encaa.pdf. [ Links ]
12. Finkelstein EA, Trgdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009; 28: w822-w831. [ Links ]
13. Winter AC, Berger K, Buring JE, Kurth T. Body mass index, migraine, migraine frequency and migraine features in women. Cephalalgia 2009; 29: 269-78. [ Links ]
14. Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: a population study. Neurology 2006; 66: 545-50. [ Links ]
15. Mattsson P. Migraine headache and obesity in women aged 40-74 years: a population-based study. Cephalalgia 2007; 27: 877-80. [ Links ]
16. Bigal ME, Tsang A, Loder E, Serrano D, Reed ML, Lipton RB. Body mass index and episodic headaches: a population-based study. Arch Intern Med 2007; 167: 1964-70. [ Links ]
17. Peterlin BL, Rosso AL, Rapoport AM, Scher AI. Obesity and migraine: the effect of age, gender and adipose tissue distribution. Headache 2010; 50: 52-62. [ Links ]
18. Taylor FR. Weight change associated with the use of migraine-preventive medications. Clin Ther 2008; 30: 1069-80. [ Links ]
19. Tremblay A, Chaput JP, Bérubé-Parent S, et al. The effect of topiramate on energy balance in obese men: a 6 month doubleblind randomized placebo-controlled study with a 6 month open-label extension. Eur J Clin Pharmacol 2007; 63: 123-34. [ Links ]
20. Stewart WF, Lipton RB, Whyte J, et al. A multinational study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology 1999; 53: 988-94. [ Links ]
21. ABEP - Associação Brasileira de Empresas de Pesquisa. Critério de Classificação Econômica Brasil (document on the Internet). 2011 (cited 2011 April 06). Available from: http://www.abep.org. [ Links ]
22. WHO-World Health Organization. Obesity: Preventing and managing the global epidemic - Report of a WHO consultation on obesity (document on the Internet). 2000 (cited 2013 Feb 07). Available from: http://www.bvsde.paho.org/bvsacd/cd66/obeprev/indice.pdf. [ Links ]
23. Lipschitz DA. Screening for nutritional status in the elderly. Prim Care 1994; 21: 55-67. [ Links ]
24. WHO-World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of WHO Expert Consulation (document on the Internet). 2008 (cited 2013 Feb 07). Available from: http://whqlibdoc.who.int/publications/2011/9789241501491_eng.pdf. [ Links ]
25. Fukui PT, Gonçalves TRT, Strabeli CG, et al. Trigger Factors in migraine patients. Arq Neuropsiquiatr 2008; 66 (3-A): 494-9. [ Links ]
26. Rockett FC, Kastro K, Oliveira VR, Perla AS, Chaves MLF, Perry IDS. Perceived migraine triggers: Do dietary factors play a role? Nutr Hosp 2012; 27 (2): 483-9. [ Links ]
27. Friedman DI, De ver dye T. Migraine and the Environment. Headache 2009; 49 (6): 941-52. [ Links ]
28. Horev A, Wirguin I, Lantsberg L, Ifergane G. A high incidence of migraine with aura among morbidly obese women. Headache 2005; 45 (7): 936-8. [ Links ]
29. Bond DS, Roth J, Nash JM, Wing RR. Migraine and obesity: epidemiology, possible mechanisms and the potential role of weight loss treatment. Obes Rev 2011; 12: 362-71. [ Links ]
30. Bond DS, Vithiananthan S, Nash JM, Thomas JG, Wing RR. Improvement of migraine headaches in severely obese patients after bariatric surgery. Neurology 2011; 76: 1135-8. [ Links ]
31. Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 2001; 9: 414-7. [ Links ]
32. Beda RD, Gill EA Jr. Patent foramen ovale: does it play a role in the pathophysiology of migraine headache? Cardiol Clin 2005; 23: 91-6. [ Links ]
33. Stang PE, Carson AP, Rose KM et al. Headache, cerebrovascular symptoms, and stroke: The Atherosclerosis Risk in Communities Study. Neurology 2005; 64: 1573-7. [ Links ]
34. Kurth T, Slomke MA, Kase CS et al. Migraine, headache, and the risk of stroke in women. Neurology 2005; 64: 1020-6. [ Links ]
35. Young WB, Rozen TD. Preventive treatment of migraine: effect on weight. Cephalalgia 2005; 25: 1-11. [ Links ]
36. Maggioni F, Ruffatti S, Dainese F, Mainardi F, Zanchin G. Weight variations in the prophylactic therapy of primary headaches: 6-Month follow-up. J Headache Pain 2005; 6: 322-4. [ Links ]
37. Diener HC, Tfelt-Hansen P, Dahlöf C, et al. Topiramate in migraine prophylaxis- results from a placebocontrolled trial with propranolol as an active control. J Neurol 2004; 251: 943-950. [ Links ]
38. Silberstein SD, Neto W, Schmitt J et al. Topiramate in migraine prevention: Results of a large controlled trial. Arch Neurol 2004; 61: 490-5. [ Links ]
39. Dodick DW, Silberstein SD, Freitag F, et al. Topiramate versus amitriptyline for migraine prophylaxis: A multicenter, randomized, doubleblind, parallel treatment group trial. Cephalalgia 2006; 26: 1373. [ Links ]
![]() Correspondence:
Correspondence:
Ingrid Dalira Schweigert Perry
Hospital de Clínicas de Porto Alegre
Centro de Pesquisa Clínica - room 21307
Rua Ramiro Barcelos, 2350
CEP 90035-903 Porto Alegre - RS. Brazil
E-mail: atputp@gmail.com
Recibido: 14-II-2013
1.a Revisión: 21-II-2013
Aceptado: 27-III-2013.