Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.6 Madrid nov./dic. 2013
https://dx.doi.org/10.3305/nh.2013.28.6.6831
REVISIÓN
Lactobacillus Paracasei subsp. Paracasei F19; a farmacogenomic and clinical update
Lactobacillus Paracasei sybsp. Paracasei F19; una actualización de farmacogenomic y clínica
Alessandro Di Cerbo1 and Beniamino Palmieri2
1Department of Clinical Microbiology.
2Department of General Surgery and Surgical Specialties. University of Modena e Reggio Emilia. Modena. Italy.
ABSTRACT
Introduction: Many reports in literature have underlined particular features of Lactobacillus paracasei subsp paracasei F19, however a critical review of main clinical outcomes has not been performed so far.
Objectives: This review summarizes the most relevant reports, in terms of clinical benefits, of Lactobacillus paracasei subsp paracasei F19 administration reviewing it's historical background and outlining new interesting perspectives in clinical practice.
Methods: We searched Pubmed/Medline using the terms "Lactobacillus paracasei subsp paracasei F19". All clinical and experimental articles on the use of Lactobacillus paracasei subsp paracasei F19 were included.
Results and discussion: The genetic stability of F19, the most relevant clinical claim, renders it's administration reliable and effective in immunocompromised people. Adequate concentrations of this strain support a dose/effect strategy ranging between NF B host macrophage activation to pathogenic bacteria overgrowth control as well as to fine interaction with the gut nerve endings. Moreover preliminary results from our lab support the formulation of F19 encapsulated with lyophilized HA in patients with IBD due to both an increased mucous-strain adherence and a possible enhanced strain proliferation and maintenance.
Conclusions: Further experiments are required to overcome the lack of informations about this new formulation for IBD management.
Key words: Lactobacillus paracasei subsp paracasei F19. Lactic acid bacteria. Probiotic.
RESUMEN
Introducción: Muchas notificaciones en la bibliografía han puesto de manifiesto las características particulares del Lactobacillus paracasei, subespecie paracasei F19; sin embargo, la revisión crítica de los principales resultados clínicos aún no se ha realizado.
Objetivos: Esta revisión resume los artículos más relevantes, en términos de beneficios clínicos, sobre administración del Lactobacillus paracasei, subespecie paracasei F19, revisando su historia y recalcando nuevas perspectivas interesantes sobre su uso en la práctica clínica.
Métodos: realizamos una búsqueda en Pubmed/Medline usado los términos "Lactobacillus paracasei subsp paracasei F19". Se incluyeron todos los artículos experimentales que empleasen el Lactobacillus paracasei, subespecie paracasei F19.
Resultados y discusión: La estabilidad genética de F19, su característica clínica más relevante, hace que su administración sea fiable y eficaz en personas inmunosuprimidas. Las concentraciones adecuadas de esta cepa apoyan una estrategia de dosis/efecto que varía entre la activación de los macrófagos del hospedador hasta un control del sobrecrecimiento de bacterias patógenas, así como una adecuada interacción con las terminaciones nerviosas intestinales. Además, los resultados preliminares de nuestro laboratorio apoyan la formulación encapsulada de Lactobacillus F19 con AH liofilizada en pacientes con EII debido a una mayor adherencia de la cepa a la mucosa y un posible favorecimiento de la proliferación y mantenimiento de la cepa.
Conclusiones: Se necesitan experimentos adicionales para paliar el déficit de información acerca de esta nueva formulación para el tratamiento de la EII.
Palabras clave: Lactobacillus paracasei subespecie F19. Bacterias acidolácticas. Probióticos.
Abbreviations
L: Lactobacillus.
LAB: lactic acid bacteria.
F19: L. paracasei subsp paracasei F19.
H. pylori: Helicobacter pylori.
P. pentosaceus: Pediococcus pentosaceus.
L. mesenteroides: Leuconostoc mesenteroides.
C. difficile: Clostridium difficile.
B: Bifidobacterium.
EEC: enteroinvasive Escherichia coli.
HA: hyaluronic acid.
E. coli: Escherichia coli.
P. aeruginosa: Pseudomonas aeruginosa.
Introduction
The Lactobacillus casei (L. casei) group is mainly composed of lactic acid bacteria (LAB) such as Lactobacillus zeae (L. zeae), L. casei, Lactobacillus paracasei (L. paracasei) and Lactobacillus rhamnosus (L. rhamnosus) widely used in dairy products and lactic beverages and whose major end product of sugar fermentation is lactic acid. These bacteria are also gram-positive, nonsporing, catalase-negative ,devoid of cytochromes and of nonaerobic habit but are aerotolerant, unpleasant, acid-tolerant and strictly fermentative1. Studies on the electrostatic cell surface properties revealed a pH-dependent configuration with electrophoretic mobility progressively decreasing for lower pH values for the Lactobacillus casei subsp. casei (L. casei subsp. casei) and Lactobacillus paracasei subsp. paracasei (L. paracasei subsp. paracasei) strains until the isoelectric point (IEP = 4). The pH variations might be linked to cell wall chemical composition with adhesion mechanism during gastric transit2.
The taxonomic position and nomenclature of the L. casei group has been long time debated3. In the past L. casei group had been one species divided into five subspecies: the L. casei subspecies casei, alactosus, pseudoplantarum, tolerans and rhamnosus4, but in 1989 Collins et al. introduced a reclassification by introducing two new species: L. paracasei and L. rhamnosus and tranferring subspecies L. casei subspecies casei to the species L. casei without any subspecies5. L. paracasei comprised two subspecies: the subspecies Lactobacillus including L. casei subspecies alactosus and pseudoplantarum and the subspecies tolerans from the L. casei subspecies with the same name.
L. paracasei subsp paracasei F19 (F19) belongs to the homofermentative family of lactic acid bacteria which convert almost quantitatively glucose to lactic acid6. It showed the ability to bind gastric and bovine mucin, collagen I and III and fibronectin and to express high surface hydrophobicity. The survival to pH 2.5 for 1 h and 20% bile for 2 h exposure, the bacteriocin(s) production, the proteolytic activity as well as the ability the ability to transcribe NF B to the nucleus of macrophages have made this lactobacillus a reasonable candidate for a probiotic product development7.
State of the art
F19 was isolated, for the first time, from the deep colonic mucus layer of patients without gastrointestinal disease who were admitted to the Sabbatsberg Hospital of Stockholm in 20027. Then it was included in the multicentre European project PROBDEMO, which involved volunteers from Finland and Sweden, where its gastric survival ability was assessed and where strains closely related to F19 were observed in the intestinal tract of a small percentage of volunteers8.
Within the PROBDEMO project human pilot studies, based on the F19 strain, have been conducted. One involved 61 healthy swedish infants and 30 elderly that were randomly assigned to receive or gelatin capsules containing 1x1010 CFU of F19 in corn starch or corn starch only for 12 weeks9. Fecal samples from infants treatment group (n = 30), collected before, during (at 2-3 weeks) and 2 weeks after administration, revealed an increase of F19 in 6/30 and a reduction of 17% of Clostridium difficile (C. difficile) population. However the elderly treatment group (n = 13) did not evidence substantial changes in terms of both microbial colonization and, as for the infants, Helicobacter pylori (H. pylori) eradication. The overall results indicated the ability of F19 to survive through the gastrointestinal tract and in 8-20% to be enclosed in the microbiota for several weeks as a part of the normal microflora. Another trial, still based on the assessment of gastric survival and mucosal adhesion of F19, indicated that it was both in the lumen and adhered to the mucosa of the colon (0.9x104 CFU) following 12 day consumption in 5 individuals10. Potential side-effects of probiotic consumption, such as intestinal discomfort, increased flatulence and changes in stool consistency and frequency, were monitored. All studies reported no adverse effects.
A questionable issue that has been extensively studied for the F19 is the genetic stability assessment, especially in order to guarantee consumers of the quality of probiotic11-12. The stability of three extrachromosomal elements (plasmids) within F19 was carefully checked in each step of the industrial reproduction process and their genetic profile was compared with those determined in the same strain 6 years earlier. At the end of the study no selection of cured derivatives was detected and all plasmids resulted unaltered.
A limited effect of F19 on resistant isolates during treatment with penicillin and quinolones was also observed13. In particular, 20 patients (divided in 2 groups) treated with either penicillin or ciprofloxacin (or norfloxacin) were randomized to receive a placebo or a probiotic product made of powdered milk (10 g) and freeze-dried F19 (1010 CFU/ml). Fecal samples, collected before treatment on day 10 and 1 month after the start of the treatment, revealed that resistance in enterococci was not affected by penicillin administration while quinolone resistance increased during quinolone treatment. Moreover F19 was recovered in three samples from 10 patients in penicillin-treated group (2.1 x 103-5.3 x 104 CFU) and in three samples of eight patients (2.1 x 103-1.6 x 105 CFU) of the quinolone-treated group on day 10. Further, one patient of the quinolone-treated group still harboured the probiotic strain on day 30 (2.1 x 102 CFU).
The effectiveness of the contemporary oral administration of F19 (Genefilus F19, Siffra Farmaceutici, Florence, Italy) in association with vaginal suppositories containing L. acidophilus has been was tested by Delia et al14. 60 healthy women (18-40 years) with suspect or confirmed diagnosis of bacterial vaginosis were randomized to receive either vaginal suppositories containing Lactobacillus acidophilus (L. acidophilus) (Calagin, Siffra Farmaceutici, Florence, Italy) or the same vaginal suppositories plus oral administration of Genefilus F19 (12.5 x 109 CFU per sacket). The patients were examined at the end of therapy (3 months) and 3 months after the end of treatment. A significant reduction of vaginal pH, an improvement of sniff test as well as of the subjective symptomatology were observed in both groups at end of therapy and still decreased during the 3 months follow-up. However the Genefilus F19-treated group had a meaningful reduction of vaginal pH and of sniff test at the end of therapy and a maintenance of positive effect also after 3 months.
Also the host immunomodulation, a claim of many probiotics involved in normal immune function development, has been analyzed. Based on previous clinical studies15-18, a synbiotic combination of 1011 CFU of Pediococcus pentosaceus (P. pentosaceus) 5-33:3, Leuconostoc mesenteroides (L. mesenteroides) 32-77:1, F19; L. plantarum 2,362 plus inulin, oat bran, pectin, and resistant starch (Synbiotic 2000 Forte, Medipharm, Sweden)19-21, was randomly administered for 15 days (1 sachet of 12 g) whereas the placebo consisted of an identical dose of maltodextrin (Caloreen, Nestle, UK) on 65 critically ill patients21.
Analysis of infections, systemic inflammatory response syndrome, severe sepsis, and mortality, were performed. Results indicated that synbiotic-treated patients significantly reduced the rate of infections (P = 0.01), systemic inflammatory response syndrome, severe sepsis (P = 0.02), and mortality; further, hospitalization within the intensive care unit (P = 0.01) and days under mechanical ventilation were significantly reduced with respect to placebo (P = 0.001).
The role of F19 in this mix has been peculiar, it was the strongest inducer of Th1 and repressor of Th2 cytokines22 and, along with Lactobacillus plantarum (L. plantarum), was effective to eliminate C. difficile strains23. On the other hand it is worth noting that the PRONUT study evidenced that Synbiotic 2000 Forte did not improve severe acute malnutrition outcomes in 399 Malawian children24.
On the basis of microbiological evidence it has been also observed that F19 did not modify CD4 T-cells growth, responsible for normal immunomediated response, on the contrary this population was stimulated by L. paracasei subsp. paracasei B2106025.
By means of the gene array technology it has been possible to map the host global gene expression profile changes caused by both F19 and L. acidophilus NCFB 1748 administration (5 x108 CFU/ml)26. Differences in gene transcription were evaluated in the distal ileum of normal microflora and germ-free mice. In the normal microflora mice both strains caused concerted enhancement in a cluster of genes involved in immune response (such as Igh-5; Ms4a1; Clu; Cxcl13), some belonging to B cell receptor-signalling (Cd79a, Ms4a1, Cd19, Blk, Cd79b, Ptprc), some implicated in phagocytosis (Mfge8, Coro1a), in complement function (C3, Clu), in the architectural organization of B cells within lymphoid structures (Ltb, Cxcl13) and some implicated in protection against mucosal damage in inflammatory bowel disease (Serpina1) suggesting also a possible role of Lactobacilli in reducing the severity of inflammatory bowel disease. Although no qualitative differences on the expression profile of immune response-related genes were detected the mean signal increase was higher in mice fed with L. acidophilus NCFB 1748. In mice raised under germ-free conditions immune stimulatory effects were not observed obviously due to gut lymphoid tissue incompetence. Moreover, in germ-free mices fed with Lactobacilli an increased signal for adiponectin and adipsin (or factor D)27 in combination with reduced expression of resistin like β was observed.
West and coworkers determined the impact of F19 during weaning on infections and IgG antibody responses to routine vaccines in 179 infants28. 89 infants were fed cereals with F19 (treatment group) whereas 90 without F19 (placebo) from 4 to 13 months of age. All of them were immunized with diphtheria and tetanus toxoid and a cellular pertussis, polio and Hib-conjugate vaccines at 5½ and 12 months of age and the number of days with infections, antibiotic prescriptions and antibody concentrations to all vaccines before and after the second and third doses were assessed. Both groups did not differ in the days number with infectious symptoms but in days with antibiotic prescriptions (fewer in the treatment group p = 0.044); moreover F19 feeding (1 x 108 CFU/ml) enhanced anti-diphteria concentrations when adjusting for breastfeeding duration and colonization (p = 0.024). An interaction of the intervention and colonization with F19 on anti-tetanus toxoid concentrations occurred during the course of vaccination (p = 0.035). No effect exerted by F19 was observed on anti-HibPS (Polio + Hib) concentrations on infants breastfed <, > or = 6 months. Nevertheless F19 feeding increased the capacity to raise immune responses to protein antigens especially in infants breastfed < 6 months but did not prevent infections.
One year later the same group evaluated the effects of F19 feeding on the incidence of eczema and Th1/Th2 balance during weaning29. From 4 to 13 months of age 89 infants were fed cereals with F19 (1 x 108 CFU) whereas 90 were only fed cereals. As a proxy for immune balance it was used the IFN- /IL4 mRNA expression levels in polyclonally stimulated peripheral blood T cells. The cumulative incidence of eczema at 13 months was 11% and 22% in the probiotic and placebo groups, respectively (p < 0.05) and the IFN-y /IL4 mRNA ratio was higher in the probiotic compared with the placebo group (p < 0.05). Conversely, no differences between groups in serum concentrations of total or specific IgE were observed.
F19 has been confirmed to be efficacious and well tolerated in patients with IBS presenting with diarrhoea or with constipation30. In particular, 100 IBS patients both with diarrhoea (n = 52) and constipation (n = 48) were administered Genefilus F19 at a dose of one sachet (12 x l09 of F19, combined with 750 mg gluco-oligosaccharides plus vitamins Bl, B5 and B6), dissolved in water, twice daily for 14 days. Moreover the content of F19 was evaluated in the stool of 20 patients before and after treatment. 2 weeks after the end of the treatment 94% of patients with IBS with diarrhoea had no more abdominal pain and 88% had no more diarrhoea; on the other hand, abdominal pain and constipation were no longer present in 87% and 83% of patients with IBS with constipation respectively; 95% of the overall population considerably improved or eliminated abdominal distension. Microbiological evaluations of patients stool revealed a marked increase of F19 load following the treatment, with a minimum of 2, to a maximum of 10, CFU/g faeces. The same trend, although with less evidence, was observed by Simren et al (2009). 74 IBS patients were randomized to receive a daily treatment with either milk fermented with the yoghurt bacteria and containing F19, L. acidophilus La5 and Bifidobacterium lactis (B. lactis) Bb12, 5 x 107 CFU/ml, (n = 37; Cultura; active) or simply acidified milk without these bacteria (n = 37; control) for 8 weeks31. Responders were 14/37 (38%) patients in the treatment group and 10/37 (27%) patients in the control group (P = 0.3). IBS symptom severity improved significantly Both groups had an improvement of IBS symptom severity during the treatment period, in particular during the first 2 weeks.
Based on the evidence that part of the pathogenesis in chronic fatigue syndrome of the host might be due to Disturbances in intestinal microbial ecology and in the immune system32-34 Sullivan et al. (2009) evaluated the effect of F19, L. acidophilus NCFB 1748 and B. lactis Bb12, 108 CFU/ml, (Cultura Dofilus Natural Yogurt, Arla Foods, Stockholm, Sweden) on fatigue and physical activity in 15 chronic fatigue syndrome (CFS) patients35. The rationale was that lactic acid producing bacteria have been shown to prevent and alleviate gastrointestinal disturbances36 and to normalize the cytokine production37 and that such regulatory mechanism may be useful for CFS suffering patients. After 4 week of probiotic intake neurocognitive functions were improved in 6/15 patients, while no significant changes in fatigue and physical activity scores as well as in the gastrointestinal microflora were observed.
It has been observed that gut microbiota modulation, by means of probiotics intake, could be used also during obesity intervention strategies38-39.
In particular, F19 supplementation, was shown to increase the levels of lipoprotein lipase inhibitor ANGPTL4 (involved in the triglyceride deposition control into adipocytes) and peroxisome proliferator activated receptors (PPARγ and PPARα) specific targets for the treatment of type 2 diabetes and dyslipidaemia40. ANGPTL4 inhibited lipoprotein lipase action decreasing fat storage41. To investigate the effects of F19 supplementation SPF C57B/6J mice were used due to their propensity for weight gain. After 10 week treatment the serum analysis revealed that free fatty acids were not affected by the presence of F19, while the triglyceride load of the lipoprotein VLDL showed a slight but significant increase although cholesterol levels remained unchanged; on the other hand circulating ANGPTL4 levels were up-regulated and magnetic resonance imaging showed a significantly reduced body fat profile.
Recently, Nardone et al. (2010) have proposed the use of F19 as protective agent in a rat model with induced oxidative and metabolic hepatic injury (30 min ischemia and 60 min reperfusion)42. More in detail, 27 Wistar rats were fed a standard diet and 27 rats a methionine/choline deficient diet for 8 weeks before the ischemia/reperfusion procedure; within each group 7 rats were sham-operated to determine baseline conditions, 10 rats underwent I/R of the liver whereas 10 rats underwent I/R after dietary supplementation with F19 (3 x 107 CFU) for 8 weeks. After I/R rats fed a standard diet showed a decrease in sinusoid perfusion (P < 0.001), a severe liver inflammation, necrosis, an increase of tissue levels of MDA (P < 0.001), TNF-α (P< 0.001), IL-1 (P < 0.001), IL-6 (P < 0.001) as well as of serum levels of transaminase (P < 0.001) and LPS (P < 0.001) with respect to sham-operated rats. A decrease in Bacterioides, Bifidobacterium, and Lactobacillus spp (P < 0.01, P < 0.001, and P < 0.001, respectively) and an increase in Enterococcus and Enterobacteriaceae (P < 0.01 and P < 0.001, respectively) on intestinal mucosa was also observed. F19 supplementation significantly reduced the harmful effects of I/R on the liver and on gut microbiota in both groups of rats, however in methionine/choline deficient-fed rats, where the severity of liver and gut microbiota alterations were greater, a slightly less effect was observed.
Annibale et al. (2011) have successfully proposed the use of Genefilus F19 along with a high-fibre diet, for abdominal bloating and prolonged abdominal pain reduction in symptomatic uncomplicated diverticular disease43. 50 Patients were randomized to receive a high-fibre diet; 1 sachet of probiotic plus (12 x 109 CFU) a high-fibre diet (twice daily); 2 sachets of probiotic + high-fibre diet (twice daily) for 14 days/month for 6 months. Both probiotic-treated groups had a significant decrease in bloating [VAS score were 4.6 ± 2.6 (baseline) vs. 2.3 ± 2.0 (end of treatment), P < 0.05 and 3.9 ± 2.9 vs. 1.8 ± 2.1, P < 0.05 respectively for the two groups] but not a significant decrease in abdominal pain within 24 hours and < 24 hours. Notably, 7 patients belonging to the probiotic-treated groups with abdominal pain > 24 hours did not report the recurrence of this symptom whereas 3 patients of the high-fibre diet- treated group reported at least one episode (P = 0.016).
A recent study has highlighted the potential use of F19 in NEC Bell's stage 2 (the most common acquired acute gastrointestinal illness in the neonatal period that affects about 5% of infants with birthweight <1,500 g and that is characterized by abdominal distension, bloody stools and pneumatosis intestinalis) in order to prevent the clinical progression to stage 344. 32 infants with birth weight 600 to 1500 g were randomly assigned to receive either a 5 ml probiotic supplementation (n = 18; F19; 6 x 109 CFU/day for 21 days) or standard medical treatment (n = 14). F19 supplementation was associated with lower progression to stage 3 (P < 0.05), lower mortality rate and shorter hospital stay (P < 0.05). Moreover none of probiotic-treated patients presented either sepsis or intestinal complications such as diarrhea.
The hypothesis that enteric glial cells might participate in host-bacteria cross-talk has been evaluated by Turco et al. (2013)45. Primary cultures of human enteric glial cells have been exposed both to live and heat-killed pathogenic enteroinvasive Escherichia coli (EEC) and probiotic (F19; 3.4 x 108 CFU/ml) bacteria. Results indicated that EEC activated enteric glial cells inducing the cFos and MHC II expression. After 6h exposure TLR1, TLR3 and TLR4 mRNA expression was significantly up-regulated by both EEC and F-19 (p < 0.01) with respect to the basal level. On the other hand, EEC induced a higher TLR3 expression (p < 0.01) and a significantly lower expression of TLR5 and TLR7 (p < 0.01) with respect to F19. After 24 hours exposure TLR7, TLR9 and TLR5 mRNA expression was significantly up-regulated only by F19 (p < 0.01) with respect to the basal level. Moreover TLR2 expression was significantly up-regulated by both EEC and F19 (p < 0.01) with respect to the basal level, however TLR3 expression was significantly up-regulated only by EEC (p < 0.01) and conversely TLR7, TLR9 and TLR5 mRNA expression was significantly up-regulated only by F19 (p < 0.01) with respect to the basal level. Notably, EEC induced a significantly higher expression of TLR2, TLR3, TLR7 and TLR9 (p < 0.01) and a lower TLR5 expression (p < 0.01) with respect to F19.
When enteric glial cells were challenged for 6 h with either heat-inactivated EEC or F19, TLR2, TLR7 and TLR9 expression was virtually undetectable with respect to the basal level while TLR5 expression was significantly down-regulated by heat-inactivated EEC (p < 0.01). After 24 h challenge, heat-inactivated EEC significantly up-regulated TLR3 expression (p < 0.01) with respect to the basal level whereas both heat-inactivated EEC and F19 up-regulated TLR4 expression (p < 0.01). The analysis of differences between viable and heat-inactivated EEC and F-19 revealed that after 6 h, but not 24 h, challenge TLR expression induced by viable organisms was significantly different from heat-inactivated ones.
Interestingly, immunofluorescence analysis showed that TLR2 was mainly detected in the cytoplasm and in the plasma membrane of enteric glial cells while TLR3 and TLR4 were mainly cytosolic and nuclear. Moreover, western blot analysis of enteric glial cells showed that EEC, but not F19, induced nuclear translocation of NF Bp50 protein (p < 0.05) with respect to the basal level as well as TLR2, TLR3 and TLR4 agonists (p < 0.05). Conversely, when enteric glial cells were treated with the specific MyD88-blocking peptide, only the TLR3 agonist significantly increased NFκBp50 expression (p < 0.05) with respect to the basal level while TLR2 and TLR4 agonists failed to induce NFκBp50 nuclear translocation. Finally, after 24 h exposure to both viable EEC and F19, S100B protein expression (and consequently NO release) was significantly higher in response to the first one (p < 0.01) compared to basal conditions. This study emphasizes both the aspect that enteric glial cells express TLR (involved in the innate immune system response mechanism)38 and their role in discriminating between pathogens and probiotics by modulating TLR expression. More recently Palumbo et al. (personal comunication) have further characterized the enteric glial cells - F19 interaction evaluating the effects of mediators released by these cells after probiotic challenge by means of conditioned media analysis46. In particular, conditioned media from probiotic stimulated cells showed increased lactase activity as compared to the untreated ones (1.15 ± 0.17 and 1.29 ± 0.19 fold increase vs control p < 0.05). However, a decreased lactase activity was observed when enteric glial cells were treated with pathogens (0.85 ± 0.23 fold decrease vs control p < 0.05).
F19: in vitro study of a potential new prebiotic enhancing activity
The concept of a possible combined administration of F19 with some new prebiotic enhancer, induced our research group to focus on the lactobacilli interaction with hyaluronic acid (HA), a large linear glycosamino-glycan which is mostly present within extracellular matrix47, that in previous microbiological and virological investigations had shown some definite properties in controlling the pathogenic bacteria and viruses growth48.
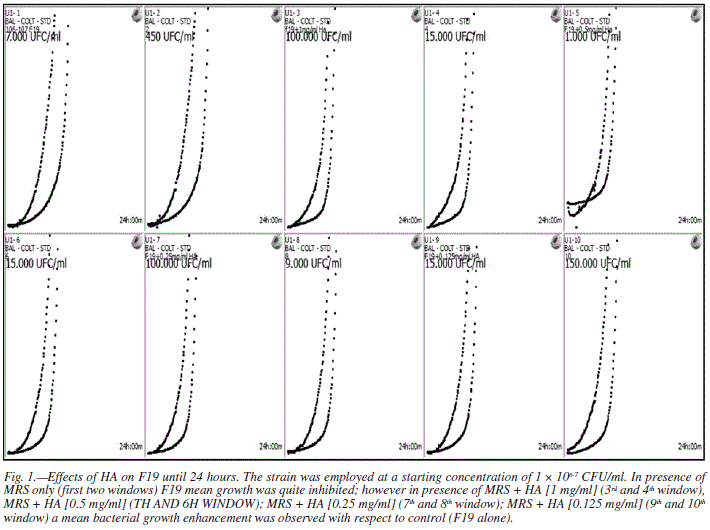
The role of HA on in vitro growth rate of F19 was investigated by means of an innovative technique. The procedure consisted in introducing, in 10 sterile vials already filled with an eugonic broth, 106-7 CFU/ml of F19 plus a decreasing concentration of HA (from 1 to 0.125 mg ml-1) and placing these in a bacterial culture (HB<M; Alifax, Padova, Italy). HB<M was used for the real time detection of bacteria growth curves. such analyzer is a new combination of a turbidimeter (based on the dynamic light scattering mechanism), with Mc Farland Monitor and an incubator within the same device and, for the first time, successfully used to accelerate studies on lactobacillus strain growth investigations. Each sample is analyzed by two laser beams which have their own photodetector, one more sensible (placed at 30o with respect to the beam source) and one more specific (placed at 90o with respect to the beam source).
With this investigation diagnostic device we demonstrated that HA concentration, from 1 to 0.125 mg ml-1, resulted in an increased bacterial strains growth within 24 hours (fig. 1). Observations might suggest a possible protective role of low doses of HA towards F19, supporting its in vivo proliferation and engraftment after oral administration.
Although speculative, a possible role of HA on the bacterial growth and proliferation might be ascribed to the ability of some strains of utilizing HA as a carbon source.
Discussion
The issue of a single lactobacillus administration compared with a pool in the probiotic therapy scenario has been debated long time without a definite indication: the concept that links each single microbiologic agent to a well defined biochemical or genetic interaction within the host environment is a clear cut premise to achieve the goal of an effective medical treatment with some sort of pharmacological approach in microbiology.
Very often the production and market of a mixed lactobacilli combination in nutraceutical products did not previously take into account the in vitro interaction between each other strain, and the impact of a stranger microbiological task force intrusion into the gut environment, doesn't guarantee at all a quicker integration or a better balance of different pathogenic versus saprophytic populations.
The bioavailability of a single strain formulation, like in the case of F19 in adequate concentration, supports a dose/effect strategy ranging between NFκB macrophage activation plus immunity host modulation, and the success over pathogenic bacteria overgrowth moreover the F19 fine interaction with the gut nerve endings, as it has been demonstrated by the in vitro experiment of Turco et al. achieves convincing explanation of the clinical healthy effect on IBD symptoms during nutraceutical treatment with this lactobacillus. Other experimental investigations opened the hypothesis that F19 displays some metabolic activating properties in the lipid imbalance and in the liver impaired functions.
Last, but not least, the genetic stability of the strain is a first class safety clinical claim, especially when probiotic therapy is required in very weak, immunocompromised people, with altered mucous barrier and with the real risk of lymphohematogenous spread of the bacteria into the bloodstream.
The term bacterial translocation, coined by Berg and Garlington49, has been defined as the passage of both viable and non-viable microbes and microbial products (endotoxins) from the intestinal lumen through the epithelial mucosa into the mesenteric lymph nodes and then to other organs. It might be related to a decrease in microbes turnover rather than an increase in their transepithelial penetration and those which seem to translocate most readily hold the ability to both survive in the bloodstream and to resist leucocyte digestion and phagocytic killing (e.g. Salmonella species). On the contrary, normal enteric species are easily killed after phagocytosis (whit the only exception of Escherichia coli (E. coli), other enterobacteriaceae, Pseudomonas aeruginosa (P. aeruginosa), enterococci and some streptococci50, surviving only under circumstances in which host defences are impaired. Recent findings have ascribed the ability of translocate of these specific strains to a better adherence and facilitated attachment to the mucus-epithelium layer with respect to nonpathogenic strains51. Although intestinal anaerobic bacteria (such as Lactobacilli) exceed aerobic bacteria by 100:1 to 1000:1 and act as an insulating layer over the mucous on the mucosal surface, limiting colonization and overgrowth of other potentially invasive microbes52, their translocation has been reported only in extreme circumstances such as athymic53, lethally irradiated54 or severely burned rodents50 and in all these conditions there are breaks in enteric integrity and the bacterial translocation appears to be in direct proportion to the degree of tissue damage. The fact that the aerobic Gram-negative bacilli can translocate even across histologically intact intestinal epithelium52 (through morphologically intact enterocytes55) and that selective elimination of anaerobic bacteria facilitates intestinal accumulation and translocation of facultative bacteria, has led to the assumption that bacterial overgrowth might be one of the main factors (reduced gastric acidity56, impaired gastrointestinal motility and, consequently, prolonged intestinal transit time57 promoting bacterial translocation. However bacterial translocation can occur in the normal host, shown by recovery o viable intestinal bacteria from mesenteric lymph nodes in a small proportion of healthy animals and humans58-59 and is a probably normal and essential process, regulating local and systemic immunity and tolerance to the innumerable antigens that make contact with the intestinal epithelium60. Usually, enteric bacteria translocation by oral antibiotics displacement remains confined to the satellite mesenteric lymph nodes and do not appear to extend a persistent infection state, moreover when the antibiotic is discontinued the caecal population of enteric organisms returns to normal levels61-62. In this context, however, immunosuppression can activate the translocating bacteria to spread systemically, ultimately resulting in lethal sepsis63. Moreover, the lower part of the gut, which contains a large number of microbes, has been suggested to hold a more efficient capacity for killing translocated bacteria with respect to the upper part; in particular the colon, which has been demonstrated to have higher electrical resistance and lower permeability to the passive movement of ions64. The lactobacilli translocation is a not uncommon relevant event that might rise pathogenic complications and even septic death: the genetic stability of F19, confirmed in more than 20 years of clinical use, not only is reassuring that the up date risk of spontaneous mutation is not consistent, but even in case of hematogenous spread of F19, it will maintain its proper immunomodulating activity; thus rendering the invaded host more active in the cell-mediated defence against the septic agents, but also it can be easily destroyed by the standard antibiotic dosages, being it's antibiogram still unchanged since 20 years.
A final comment about the F19 high daily concentration during the oral intake: being F19 acid-bile resistant it's transit through the proximal gastrointestnal tract leaves a great number of lactobacilli viable for the colonic harboring and able to survive in the colonic environment for at least three months after 4 weeks of oral intake. This means a prolonged therapeutic effect and a better temporary integration in the host microbiota related to the length of F19 administration with the confidence that the high lactobacilli count pro dose is without untoward effects (bloating, constipation or other common symptoms observed during probiotic administration, were not detected during F19 oral intake inducing an excellent compliance by the users.
Summarizing, based on recent achievements, F19, provided of genetical stability, actively interacts with gut epithelium and immune system, correlating with both gene sequences and genes whose down-regulation may be the cause of gastrointestinal pathologies.
Our present preclinical investigation addresses us to perform a next pilot study to administer F19 encapsulated with lyophilized HA in patients with IBD, in comparison with the existing formulation, in order to evaluate further symptomatic benefits due to the gut mucosa-lactobacillus interaction with a better mucous germ adherence, and possible enhanced F19 proliferation and bioavailability into the gut lumen.
As matter of fact, an other our previous unpublished study on the HA administration by enemas on a group of patients with ulcerative colitis under Pentasa treatment showed a remarkable symptomatic benefit on the number of stool-mucous discharges, bloating and pain.
We have thus a rationale to suppose that further benefit will be achieved by the synergy between probiotic and glycosaminoglycan administration. Furthermore F19 as a single therapeutic agent we'll more easily identify further specific benefits not only in the bowel inflammatory and motion control, but also in other indications related to it's metabolism in the commensal environment such as obesity and steatosis, during glyco-lipidic imbalance of diabetes, potential treatment.
References
1. Axelsson L. Lactic acid bacteria: classification and physiology. In: Salminen S, Von Wright A, eds. Lactic acid bacteria: microbiology and functional aspects (1998), New York: Marcel Dekker Inc. [ Links ]
2. Pelletier C, Bouley C, Cayuela C, Bouttier S, Bourlioux P, Bellon-Fontaine MN. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl Environ Microbiol 1997; 63 (5): 1725-31. [ Links ]
3. Sato H, Torimura M, Kitahara M, Ohkuma M, Hotta Y, Tamura H. Characterization of the Lactobacillus casei group based on the profiling of ribosomal proteins coded in S10-spc-alpha operons as observed by MALDI-TOF MS. Syst Appl Microbiol 2012; 35 (7): 447-54. [ Links ]
4. Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. Int J Food Microbiol 1998; 41 (2): 103-25. [ Links ]
5. Collins MD, Phillips BA, and Zanoni P. Deoxyribonucleic Acid Homology Studies of Lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov., comb. nov. Int J Syst Bacteriol 1989; 32 (2): 105-8. [ Links ]
6. Stolaki M, De Vos WM, Kleerebezem M and Zoetendal EG. Lactic Acid Bacteria: Microbiological and Functional Aspects (2011). [ Links ]
7. Ljungh Å, Lan J, and Yanagisawa N. Isolation, Selection and Characteristics of Lactobacillus paracasei subsp. paracasei F19. Microb Ecol Health Dis 2002; 3: 4-6. [ Links ]
8. Crittenden R, Saarela M, Mättö J, Ouwehand AC, Salminen S, Pelto L, Vaughan EE, de Vos WM, von Wright A, Fondén R and Mattila-Sandholm T. Lactobacillus paracasei subsp paracasei 19: Survival, Ecology an Safety in the Human Intestinal Tract - A Survay of Feeding Studies within the PROBDEMO Project. Microb Ecol Health Dis 2002; 3: 22-6. [ Links ]
9. Sullivan Å, Bennet Rutger, Viitanen M, Palmgren A-C, Nord CE. Influence of Lactobacillus F19 on Intestinal Microflora in Children and Elderly Persons and Impact on Helicobacter pylori Infections. Microb Ecol Health Dis 2002; 3: 17-21. [ Links ]
10. Cesena C, Morelli L, Alander M, Siljander T, Tuomola E, Salminen S, Mattila-Sandholm T, Vilpponen-Salmela T, von Wright A. Lactobacillus crispatus and its nonaggregating mutant in human colonization trials. J Dairy Sci 2001; 84 (5): 1001-10. [ Links ]
11. Morelli L, Campominosi E. Genetic stability of Lactobacillus paracasei subsp. paracasei F19. Microb Ecol Health Dis 2002; 3: 14-6. [ Links ]
12. Lombardo L. New insights into Lactobacillus and functional intestinal disorders. Minerva Gastroenterol Dietol 2008; 54 (3): 287-93. [ Links ]
13. Sullivan A, Johansson A, Svenungsson B, Nord CE. Effect of Lactobacillus F19 on the emergence of antibioticresistant microorganisms in the intestinal microflora. J Antimicrob Chemother 2004; 54 (4): 791-7. [ Links ]
14. Delia A, Morgante G, Rago G, Musacchio MC, Petraglia F, De Leo V. Effectiveness of oral administration of Lactobacillus paracasei subsp. paracasei F19 in association with vaginal suppositories of Lactobacillus acidofilus in the treatment of vaginosis and in the prevention of recurrent vaginitis. Minerva Ginecol 2006; 58 (3): 227-31. [ Links ]
15. Tok D, Ilkgul O, Bengmark S, Aydede H, Erhan Y, Taneli F, Ulman C, Vatansever S, Kose C, Ok G. Pretreatment with pro-and synbiotics reduces peritonitis-induce lung injury in rats. J Trauma 2007; 62 (4): 880-5. [ Links ]
16. Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, Bengmark S, Neuhaus P. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation-a randomized, double-blind trial. Am J Transplant 2005; 5 (1): 125-30. [ Links ]
17. Oláh A, Belágyi T, Pótó L, Romics L Jr, Bengmark S, Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology 2007; 54 (74): 590-4. [ Links ]
18. Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. J Parenter Enteral Nutr 2007; 31 (2): 119-26. [ Links ]
19. Koutelidakis IM, Bezirtzoglou E, Giamarellos-Bourboulis EJ, Grosomanidis V, Kotzampassi K. Impact of synbiotics on the intestinal flora of critically ill patients with multiple injuries. Int J Antimicrob Agents 2010; 36 (1): 90-1. [ Links ]
20. Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma 2009; 67 (4): 815-21. [ Links ]
21. Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg 2006; 30(10): 1848-55. [ Links ]
22. Fujiwara D, Inuoe S, Wakabayashi H, Fujii T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int Arch Allergy Immunol 2004; 135 (3): 205-15. [ Links ]
23. Naaber P, Smidt I, Stsepetova J, Brilene T, Annuk H, Mikelsaar, M. Inhibition of Clostridium difficile strains by intestinal Lactobacillus species. J Med Microbiol 2004; 53 (Pt 6): 551-6. [ Links ]
24. Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, Bahwere P, Collins S. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet 2009; 374 (9684): 136-44. [ Links ]
25. Peluso I, Fina D, Caruso R, Stolfi C, Caprioli F, Fantini MC, Caspani G, Grossi E, Di Iorio L, Paone FM, Pallone F, Monteleone G. Lactobacillus paracasei subsp. paracasei B21060 Suppresses Human T-Cell Proliferation. Infect Immun 2007; 75(4): 1730-7. [ Links ]
26. Nerstedt A, Nilsson EC, Ohlson K, Håkansson J, Thomas Svensson L, Löwenadler B, Svensson UK, Mahlapuu M. Administration of Lactobacillus evokes coordinated changes in the intestinal expression profile of genes regulating energy homeostasis and immune phenotype in mice. Br J Nutr 2007; 97 (6): 1117-27. [ Links ]
27. Loyet KM, Deforge LE, Katschke KJ Jr, Diehl L, Graham RR, Pao L, Sturgeon L, Lewin-Koh SC, Hollyfield JG, van Lookeren Campagne M. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci 2012; 53 (10): 6628-37. [ Links ]
28. West CE, Gothefors L, Granström M, Käyhty H, Hammarström ML, Hernell O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr Allergy Immunol 2008; 19 (1): 53-60. [ Links ]
29. West CE, Hammarström ML, Hernell O. Probiotics during weaning reduce the incidence of eczema. Pediatr Allergy Immunol 2009; 20 (5): 430-7. [ Links ]
30. Lombardo L, Vernetto A, Blanco I. Clinical evaluation of Lactobacillus paracasei subsp. paracasei F19 with gluco-oligosaccharides in the short-term treatment of irritable bowel syrndrome. Microb Ecol Health Dis 2009; 21: 28-32. [ Links ]
31. Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther 2010; 31 (2): 218-27. [ Links ]
32. Evengård B, Klimas N. Chronic fatigue syndrome: probable pathogenesis and possible treatments. Drugs 2002; 62 (17): 2433-46. [ Links ]
33. Adler RH. Chronic fatigue syndrome (cfs). Swiss Med Wkly 2004; 134 (19-20): 268-76. [ Links ]
34. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002; 122 (4): 1140-56. [ Links ]
35. Sullivan A, Nord CE, Evengård B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J2009; 8 (4). [ Links ]
36. Cross ML, Gill HS. Can immunoregulatory lactic acid bacteria be used as dietary supplements to limit allergies? Int Arch Allergy Immunol 2001; 125 (2): 112-9. [ Links ]
37. Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 1997; 99 (2): 179-85. [ Links ]
38. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444 (7122): 1027-31. [ Links ]
39. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 2009; 457 (7228): 480-4. [ Links ]
40. Yoon JC, Chickering T, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 2000; 20 (14): 5343-9. [ Links ]
41. Aronsson L, Huang Y, Parini P, Korach-André M, Håkansson J, Gustafsson JÅ, Pettersson S, Arulampalam V, Rafter J. Decreased Fat Storage by Lactobacillus Paracasei Is Associated with Increased Levels of Angiopoietin-Like 4 Protein (ANGPTL4). PLoS One 2010; 5 (9). [ Links ]
42. Nardone G, Compare D, Liguori E, Di Mauro V, Rocco A, Barone M, Napoli A, Lapi D, Iovene MR, Colantuoni A. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am J Physiol Gastrointest Liver Physiol 2010; 299 (3): G669-76. [ Links ]
43. Annibale B, Maconi G, Lahner E, De Giorgi F, Cuomo R. Efficacy of Lactobacillus paracasei sub. paracasei F19 on abdominal symptoms in patients with symptomatic uncomplicated diverticular disease: a pilot study. Minerva Gastroenterol Dietol 2011; 57 (1): 13-22. [ Links ]
44. Zampieri N, Pietrobelli A, Biban P, Soffiati M, Dall'Agnola A, Camoglio FS. Lactobacillus paracasei subsp. paracasei F19 in Bell's Stage 2 of necrotizing enterocolitis. Minerva Pediatr 2013; 65. [ Links ]
45. Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D'Alessandro A, Cammarota M, Giuliano M, Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2013. [ Links ]
46. DDW 2013-Digestive Disease Week- Orange County Convention Center - Orlando (Florida); May 18-21. [ Links ]
47. Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol 2003; (3): 143-53. [ Links ]
48. Ardizzoni A, Neglia R, Baschieri MC, Cermelli C, Caratozzolo M, Righi E, Palmieri B, Blasi E. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J Mater Sci Mater Med 2011; 22 (10): 2329-38. [ Links ]
49. Berg RD, Garlington A. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979; 23 (2): 403-11. [ Links ]
50. Wells CL. Relationship between intestinal microecology and the translocation of intestinal bacteria. Antonie Van Leeuwenhoek 1990; 58 (2): 87-93. [ Links ]
51. Ljungdahl M, Lundholm M, Katouli M, Rasmussen I, Engstrand L, Haglund U. Bacterial translocation in experimental shock is dependent on the strains in the intestinal flora. Scand J Gastroenterol 2000; 35 (4): 389-97. [ Links ]
52. Wells CL, Maddaus MA, Reynolds CM, Jechorek RP, Simmons RL. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun 1987; 55 (11): 2689-94. [ Links ]
53. Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun 1980; 27 (2): 461-7. [ Links ]
54. Brook I, MacVittie T. Walker RI. Recovery of aerobic and anaerobic bacteria from irradiated mice. Infect Immun 1984; 46 (1): 270-1. [ Links ]
55. Alexander JW, Boyce S, Babcock GF, Giannotti L, Peck MD, Dunn DL, Pyles T, Childress CP, and Ash SK. The process of microbial translocation. Ann Surg 1990; 212: 496-510. [ Links ]
56. Stockbruegger RW. Bacterial overgrowth as a consequence of reduced gastric acidity. Scand J Gastroenterol Suppl 1985; 111: 7-16. [ Links ]
57. Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg 1998; 228 (2): 188-93. [ Links ]
58. Sedman PC, Macfie J, Sagar P, Mitchell CJ, May J, Mancey-Jones B, Johnstone D. The prevalence of gut translocation in humans. Gastroenterology 1994; 107 (3): 643-9. [ Links ]
59. Brooks SG, May J, Sedman P, Tring I, Johnstone D, Mitchell CJ, MacFie J. Translocation of enteric bacteria in humans. Br J Surg 1993; 80 (7): 901-2. [ Links ]
60. Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol 2003; 17 (3): 397-425. [ Links ]
61. Berg RD, Owens W. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect Immun 1979; 25(3): 820-7. [ Links ]
62. Berg RD. Mechanisms confining indigenous bacteria to the gastrointestinal tract. Am J Clin Nutr 1980; 33 (11 Supl.): 2472-84. [ Links ]
63. Berg RD, Wommack E, Deitch EA. Immunosuppression and intestinal bacterial overgrowth synergistically promote bacterial translocation. Arch Surg 1988; 123 (11): 1359-64. [ Links ]
64. Powell DW. Barrier function of epithelia. Am J Physiol 1981; 241 (4): G275-88. [ Links ]
![]() Correspondence:
Correspondence:
Alessandro Di Cerbo.
E-mail: alessandro811@hotmail.it
Recibido: 22-V-2013.
1.a Revisión: 3-VII-2013
Aceptado: 18-IX-2013.