Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.6 Madrid nov./dic. 2013
https://dx.doi.org/10.3305/nh.2013.28.6.6911
ORIGINAL / Obesidad
Central obesity measurements predict metabolic syndrome in a retrospective cohort study of postmenopausal women
Obesidad abdominal determinada por DEXA como predictor de síndrome metabólico en mujeres postmenopáusicas
Manuel Rosety-Rodríguez1, Gabriel Fornieles1, Ignacio Rosety4, Antonio J. Díaz1, Miguel A. Rosety4, Alejandra Camacho-Molina3, A. Rodríguez-Pareja5, A. Tejerina2, José Ramón Alvero-Cruz6 and Francisco J. Ordonez4
1Department of Medicine. School of Medicine. University of Cadiz (Cadiz, Spain).
2Division of Radiology. Foundation Jimenez Diaz: Autonomous University Madrid. (Madrid, Spain).
3Division of Internal Medicine. Juan Ramon Jimenez Hospital. (Huelva, Spain).
4Department of Human Anatomy. School of Medicine. University of Cadiz (Cadiz, Spain).
5Division of Gynecology. Jerez University Hospital. (Cadiz, Spain).
6Spanish Group of Kineanthropometry. School of Medicine. University of Malaga (Malaga, Spain).
ABSTRACT
Introduction: The various diagnostic classifications in the literature concur as regards the important role of abdominal obesity in the onset and progression of metabolic syndrome. Accordingly, this study was aimed at clarifying whether central obesity measurements assessed by dual X-ray absorptiometry (DXA) may predict metabolic syndrome in Spanish postmenopausal women.
Material and methods: This historical cohort study included a total of 1326 postmenopausal women aged > 45 years old who had routinely undergone DXA to measure their bone mineral density between january 2006 and january 2011. The regions of interest (ROI) envisaged in our study by using DXA were the lumbar regions L1-L4 and L4-L5. At the same time, they underwent a complete medical examination including personal medical history assessment, biochemical blood analysis, blood pressure measurement and anthropometrical evaluation. Metabolic syndrome was diagnosed attending to the criteria established by National Cholesterol Education Program Adult Treatment Panel III (NECP-ATP-III).
Results: During the observation period, 537 women, representing 40.5% of the total studied, met the diagnostic criteria for metabolic syndrome. L1-L4 and L4-L5 abdominal fat mass determinations were associated with the development of metabolic syndrome in all regression models tested, showing an increasing gradient from the lowest to highest quintile.
Conclusion: Central adiposity measurements assessed by DXA, especially L1-L4 region of interest, could be considered a powerful predictor of metabolic syndrome in postmenopausal women.
Key words: Metabolic syndrome. Abdominal obesity. Postmenopause.
RESUMEN
Introducción: En la actualidad se acepta la importancia de la masa grasa abdominal en la fisiopatología del síndrome metabólico tal y como reconocen las diferentes clasificaciones diagnósticas disponibles. Nuestro objetivo fue analizar la utilidad como predictores de síndrome metabólico de marcadores de grasa abdominal obtenidos por DEXA en mujeres postmenopausicas aprovechando su participación en screening rutinarios para el estudio de la densidad mineral ósea.
Material y método: El presente estudio de cohortes histórico incluyó a un total de 1.326 mujeres post-menopausicas con edad > 45 años que se habían sometido rutinariamente a DEXA para conocer su densidad mineral ósea entre enero de 2006 y enero de 2011. Además del DEXA, se obtuvo de cada participante la correspondiente anamnesis, bioquímica, tensión arterial e índices de distribución de masa grasa mediante técnicas antropométricas convencionales. Se utilizó la clasificación NCEP-ATP-III para el diagnóstico de síndrome metabólico. Este protocolo fue aprobado por un Comité de Ética Institucional.
Resultados: Durante el periodo de observación, 537 mujeres, el 40,5% del total de las estudiadas, cumplió los criterios diagnósticos de síndrome metabólico. Los parámetros de masa grasa abdominal obtenidos mediante DEXA fueron significativamente mayores en mujeres postmenopáusicas con síndrome metabólico. Finalmente, la masa grasa abdominal de regiones de interés L1-L4 y L3-L4 obtenidas por DEXA se relacionaron con el desarrollo de síndrome metabólico en los modelos de regresión ensayados.
Conclusión: La masa grasa abdominal determinada por DEXA, especialmente la región L1-L4, podría recomendarse como predictor de síndrome metabólico en este grupo.
Palabras clave: Síndrome metabólico. Obesidad abdominal. Postmenopausia.
Introduction
Metabolic syndrome is receiving increasing attention in the specialist literature, partly because of the high morbidity, mortality and health care costs associated with this condition1. Furthermore, prevalence of the syndrome is increasing, especially among women, reaching 45.2% in a stratified random sample of 3915 adult women from different Spanish regions2.
Although there is unanimous agreement on the importance of early diagnosis in order to implement early interventions and prevent complications, there is still no consensus in the literature regarding the preferential use of any of the different diagnostic criteria for metabolic syndrome3. A recent study concluded that these definitions performed better in women than in men4.
It should be also emphasized the determination of abdominal fat mass forms part of all diagnostic classifications due to its importance in the origin and progression of the syndrome5-6.
Researchers and health professionals may benefit from technological advances that have increased available options for estimating fat mass. For some years, DXA has been used to measure bone mineral density, especially in postmenopausal women, with the aim of preventing and/or monitoring osteoporosis7. More recently, it has proven useful in the evaluation of abdominal or central fat mass thanks to the systematisation and standardisation of regions of interest (ROI) such as upper back fat (between the 1st and 4th lumbar vertebrae) and lower back fat (between the 4th and 5th lumbar vertebrae)8.
This is of particular interest due to their relevance in the pathophysiology of processes such as type 2 diabetes, metabolic syndrome, etc.9,10. In fact, Leslie et al.10 obtained excellent results using abdominal fat mass parameters derived from DXA as predictors of type 2 diabetes.
Given the above, our working hypothesis was that it would be possible to obtain kineanthropometric parameters by DXA, which could be used as predictors of metabolic syndrome in postmenopausal women who routinely participate in osteoporosis screening programmes.
Materials and methods
Study population
This was a historical cohort study which included a total of 1326 postmenopausal women aged > 45 years old who had routinely undergone DXA to measure their bone mineral density between january 2006 and january 2011. At the same time, they underwent a complete medical examination including personal medical history assessment, biochemical blood analysis, blood pressure measurement and anthropometrical evaluation.
Inclusion criteria were defined as follows: woman, postmenopausal, aged > 45 years old at the time of undergoing DXA examination. Where multiple DXA records existed for the same patient, the oldest one was used. Exclusion criteria were defined as the coexistence of uncorrected thyroid diseases, due to their impact on the body composition of these patients, diabetes, ischemic heart disease, rheumatoid arthritis, chronic obstructive pulmonary disease (COPD), dementia and corticosteroid treatment for more than 90 days during the year prior to DXA examination. Metabolic syndrome was diagnosed attending to the criteria established by NCEP ATP-III11. Lastly, it should be pointed out medical records of patients were studied from an administrative healthcare database using a case record form.
Biochemical outcomes
Blood samples were collected from the antecubital vein puncture and collected by using an evacuated tube containing EDTA. The whole blood was centrifuged at 3000 rpm for 10 minutes in a clinical centrifuge. The plasma was separated and stored at -80o C until further analysis. Lipid profile (Cholesterol-LDL; Cholesterol-HDL; Triglycerides) and fasting glucose were assessed by conventional enzymatic assay method (Boehringer, Mannheim, Germany) using the Hitachi 902 Autoanalyzer (Roche Diagnostics, Germany).
Body composition
Abdominal fat mass was determined using a Lunar DPX-L type DXA. This device has been widely used in the literature because the software it uses greatly facilitates the radiologist's task of defining ROI for the study of body fat mass. The ROI envisaged in our study were the lumbar regions L1-L4 and L4-L5. To these we added the standard parameters facilitated by the software, such as the percentages of total and trunk fat mass. It is worth emphasising that the trunk includes the trunk itself and the abdomen, but excludes the pelvis. Meanwhile, the lower member includes the hip, thigh and leg. To avoid bias when delineating ROI, it is crucial that the patient is positioned correctly in parallel to the scanner table. This procedure exposes subjects to a minimum radiation of between 0.015 to 0.06 mrem, depending on the antero-posterior diameter of the person undergoing the examination, equivalent to between 1% and 10% of a chest radiograph. The following equation was used to calculate the Body Mass Index (BMI = weight (kg) / height (m)2), that was expressed as kg/m2. Height was determined with an accuracy of 0.1 cm by precision stadiometer. Body weight was assessed with an accuracy of 0.1 kg using an electronic balance. To determine waist to hip ratio (WHR), waist and hip circumferences were measured with an anthropometric tape (Holtain Ltd). Waist was measured as halfway between the costal edge and the crista. Hip was measured as the greatest circumference around the nates. It sould be pointed out these parameters were assessed according to the International Society for the Advancement of Kineanthropometry (ISAK) guidelines by a long experienced investigator who was not involved in any other aspect of the trial.
Ethics and Statistics
This research has been conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki (version, 2007). Further this protocol was approved by an Institutional Ethics Committee (University of Seville, Spain).
The results were expressed as a mean (SD) and 95% confidence intervals (95%CI). To compare the mean values of fat mass in women with and without metabolic syndrome, Student's t-test for unpaired data was used. Finally, hazard ratios and 95%CI were estimated using a Cox proportional hazard model for time to metabolic syndrome diagnosis, based on abdominal fat measurements, adjusted for BMI and age. The significance of the changes observed was ascertained to be p < 0.05.
Results
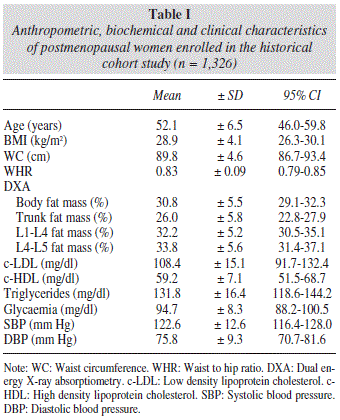
The anthropometric, biochemical and clinical characteristics of the cohort studied are summarised in table I.
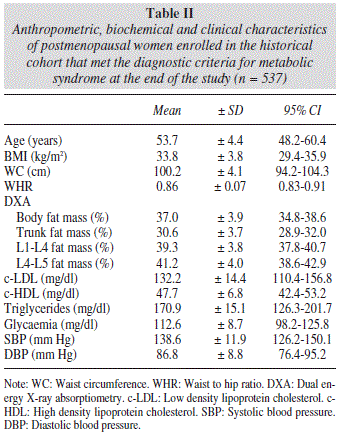
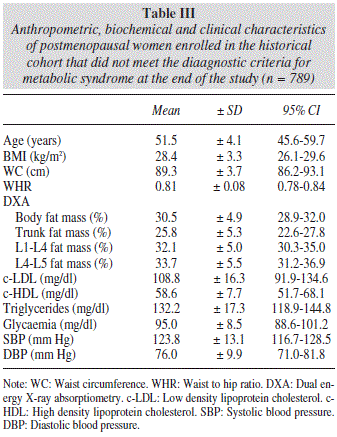
During the observation period, 537 women, representing 40.5% of the total studied, met the diagnostic criteria for metabolic syndrome. Data concerning the group of women with metabolic syndrome are listed in table II, while data on the group of women without metabolic syndrome who participated in our study are shown in table III.
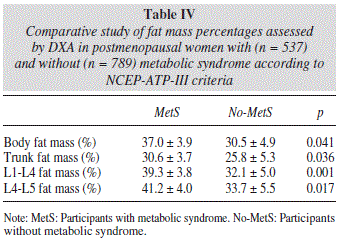
As expected, we found significant differences between the abdominal fat mass parameters obtained by DXA in postmenopausal women with and without metabolic syndrome according to the NCEP-ATP-III criteria (Table IV).
L1-L4 and L4-L5 abdominal fat mass determinations obtained by DXA were associated with the development of metabolic syndrome in all regression models tested, showing an increasing gradient from the lowest to highest quintile.
With respect to the percentage of L1-L4 fat mass, and more specifically, in models adjusted for age, women who were in the highest quintile presented a risk of developing metabolic syndrome that was 7.18 times higher (95% CI = 5.66-9.28) than women in the lowest quintile. In models adjusted for BMI, this risk was attenuated. Thus, those women who were in the highest quintile showed a risk of metabolic syndrome, which was 3.92 times higher (95% CI = 2.88-5.16) than those who were in the lowest quintile.
Similar results were obtained for L4-L5 fat mass. In models adjusted for age, the risk for women in the highest quintile was 6.92 times higher than for those in the lowest quintile (95% CI = 5.35-8.76). Meanwhile, in models adjusted for BMI, the risk for women in the highest quintile fell again, being only 3.66 times higher than that for those located in the lowest quintile (95% CI = 2.51-4.98).
Discussion
The novel contribution of this study is that by taking advantage of a routine bone mineral density screening campaign, widely accepted by professionals and patients alike12, it was possible to predict which women were predisposed to developing metabolic syndrome on the basis of their abdominal fat mass. Thus, it would be possible to take preventive measures and even implement early treatment in order to avoid complications and reduce associated health care costs. Consequently, in addition to fulfilling the criterion of originality, this study also reflects contemporary criteria insofar as it may contribute to the sustainability of the health system in the present times of budgetary constraints.
The various diagnostic classifications in the literature concur as regards the important role of abdominal obesity in the onset and progression of metabolic syndrome5. In a multicenter study carried out in 12 Latin American cities to determine metabolic syndrome risk factors according to the NCEP-ATP-III criteria, the results indicated that the greatest risk factor for post-menopausal women was obesity (OR 13.01, 95% CI, 10.93-15.49) followed at a greater distance by age (OR 1.22, 95% CI, 1.03-1.43), time since menopause (OR1.18, 95% CI, 1.00-1.38) and smoking (OR1.40, 95% CI, 1.19-1.65)13.
Abdominal fat mass is not only important in the diagnosis of metabolic syndrome but has also been proposed as a therapeutic target14. Hence, in recent years many techniques have emerged for identifying and monitoring abdominal fat mass.
Conventional kineanthropometric parameters are still used to determine abdominal fat mass due to their low cost and wide availability in any clinical setting15. However, these parameters do not differentiate between visceral and subcutaneous fat mass16.
They also present a very limited capacity for detecting small changes attributable to the implementation of weight-loss strategies such as exercise and/or low-calorie diets17. It should be pointed out that information about improvements induced intervention programs may be of particular interest for participants in order to increase their motivation and adherence to the program. Consequently, an increasing number of studies recommend the use of imaging techniques since these are more accurate and reproducible although also more costly and complex18.
The ability to determine regions of interest using DXA makes this a particularly attractive option for the early detection of abdominal fat mass before being diagnosed as obese19, especially among women20.
Previous studies have reported that the L4-L5 region or umbilicus region may not be the most predictive of morbidity21. Further, a recent study reported that the L3 or L2 region correlate as well or better than the L4-L5 region with total visceral adiposity and markers of metabolic syndrome22. Accordingly, in the present study, the L1-L4 region was more predictive of metabolic syndrome when compared to L4-L5 region in postmenopausal women.
Although other imaging techniques were not employed in this study, previous studies have found a strong correlation between levels of abdominal fat mass obtained by DXA and those recorded by computed tomography22 and magnetic resonance imaging19.
This is of great interest since DXA involves significantly less radiation than computed tomography, besides being simpler, faster and more accessible in clinical practice than magnetic resonance8,23,24. Furthermore, considering the current budget constraints, examining a patient using DXA is no more expensive than the determination of insulin resistance recommended in the WHO criteria for diagnosis of metabolic syndrome8.
Similar results have been reported for a cohort of 30.252 women over 40 years old. Specifically, the risk of developing type II diabetes in this cohort was 3.56 times higher among those who presented more abdominal fat mass than among those who were in the lowest quintile, adjusted for age, BMI and comorbidity10.
The rationale for focusing this study on post-menopausal women was that there is a higher prevalence of metabolic syndrome among this group13,25. For example, significant differences were found in the prevalence of metabolic syndrome among Spanish adults when comparing females (45.2%, 95%-CI, 43.7-46.8%) and males (33.8%, 95%-CI, 32.3-35.4%)2. Consequently, the prevalence of metabolic syndrome in this historical cohort of postmenopausal women was similar to that reported in previous studies in Spain2.
One limitation of this study was that the cohort participants were women who took part in a population-based osteoporosis screening program, and thus caution should be exercised when extrapolating the results to the general population. Nonetheless, it should be pointed out the presence of metabolic syndrome does not worsen the levels of bone mineral density in post-menopausal women. In fact, there seems to be a balance between factors that reduce bone mineral absorption (e.g. obesity) and others that increase it (low-grade chronic inflammation, hypertension)26. Furthermore, recent studies have emphasised the importance of screening for metabolic syndrome in normal weight individuals27.
Another minor limitation of this study was that the population did not include women under the age of 45 years and premenopausal. Further studies are required to determine whether age and menopause may affect these results.
Lastly, a major limitation was that DXA is a two-dimensional projection method, so within the abdominal cavity, DXA measures both the visceral adipose tissue (VAT) and the subcutaneous adipose tissue (SAT). Visceral adipose tissue (VAT) is considered to be more closely associated with obesity related diseases, such as type 2 diabetes, metabolic syndrome, etc. than other indexes of obesity28. Fortunately, Micklesfield et al.22 have already reported a more sophisticated DXA measurement of VAT. It showed a stronger correlation with VAT determined by computed tomography (CT) than could be obtained with the best anthropomorphic and demographic model. If these findings are supported by similar results in other populations, DXA may become a useful alternative to CT and magnetic resonance imaging (MRI) for the estimation of VAT in both clinical and research settings22.
It was concluded that abdominal fat mass in the L1-L4 region of interest could be considered a powerful predictor of metabolic syndrome in postmenopausal women undergoing mineral density examination by DXA. Future studies are still required to consolidate this approach, not only in the field of research but also in clinical application.
References
1. Scholze J, Alegría E, Ferri C, Langham S, Stevens W, Jeffries D, et al. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health 2010; 10: 529. [ Links ]
2. Llisterri JL, Cea-Calvo L, Martí-Canales JC, Lozano JV, Aznar J, Redón J. Prevalence of metabolic syndrome in Spanish population aged 60 years-old or more. PREV-ICTUS, a population-based study. Med Clin (Barc). 2009; 132: 172-9. [ Links ]
3. Cameron A. The metabolic syndrome: validity and utility of clinical definitions for cardiovascular disease and diabetes risk prediction. Maturitas 2010; 65: 117-21. [ Links ]
4. Lin CC, Liu CS, Li CI, Lin WY, Lai MM, Lin T, et al. The relation of metabolic syndrome according to five definitions to cardiovascular risk factors-a population-based study. BMC Public Health 2009; 9: 484. [ Links ]
5. Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039-49. [ Links ]
6. Ruano Gil M, Silvestre Teruel V, Aguirregoicoa García E, Criado Gómez L, Duque López Y, García-Blanch G. Nutrition, metabolic syndrome and morbid obesity. Nutr Hosp 2011; 26:759-64. [ Links ]
7. Moyad MA. Osteoporosis: a rapid review of risk factors and screening methods. Urol Oncol 2003; 21: 375-9. [ Links ]
8. Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 2008; 11: 566-72. [ Links ]
9. Andersson DP, Löfgren P, Thorell A, Arner P, Hoffstedt J. Visceral fat cell lipolysis and cardiovascular risk factors in obesity. Horm Metab Res 2011; 43: 809-15. [ Links ]
10. Leslie WD, Ludwig SM, Morin S. Abdominal fat from spine dual energy X-ray absorptiometry and risk for subsequent diabetes. J Endocrinol Metab 2010; 95: 3272-76. [ Links ]
11. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005; 112: 2735-52. [ Links ]
12. King AB, Fiorentino DM. Medicare payment cuts for osteoporosis testing reduced use despite tests' benefit in reducing fractures. Health Aff (Millwood). 2011; 30: 2362-70. [ Links ]
13. Royer M, Castelo-Branco C, Blümel JE, Chedraui PA, Danckers L, Bencosme A, et al. The US National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III): prevalence of the metabolic syndrome in postmenopausal Latin American women. Climacteric 2007; 10: 164-70. [ Links ]
14. Kishida K, Funahashi T, Matsuzawa Y, Shimomura I. Visceral adiposity as a target for the management of the metabolic syndrome. Ann Med 2012; 44: 233-41. [ Links ]
15. Oda E. Optimal cutoff points of waist circumference for the criteria of abdominal obesity: comparison with the criteria of the International Diabetes Federation. Circ J 2010; 74: 207. [ Links ]
16. Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the 'beef'. Int JObes (Lond). 2007; 31: 1552-3. [ Links ]
17. El Ghoch M, Alberti M, Milanese C, Battistini NC, Pellegrini M, Capelli C, et al. Comparison between dual-energy X-ray absorptiometry and skinfolds thickness in assessing body fat in anorexia nervosa before and after weight restoration. Clin Nutr 2012; 31: 911-6. [ Links ]
18. Owen K, Pettman T, Haas M, Viney R, Misan G. Individual preferences for diet and exercise programmes: changes over a lifestyle intervention and their link with outcomes. Public Health Nutr 2010; 13: 245-52. [ Links ]
19. Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord 2002; 26: 978-83. [ Links ]
20. Snijder MB, Visser M, Dekker JM, Seidell JC, Fuerst T, Tylavsky F, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with 24. computed tomography and anthropometry. Int J Obes Relat Metab Disord 2002; 26: 984-93. [ Links ]
21. Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi-Sunyer X, et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes (Lond). 2007; 31: 763-9. [ Links ]
22. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring). 2012; 20: 1109-14. [ Links ]
23. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). 2012; 20: 1313-8. [ Links ]
24. Mattsson S, Thomas BJ. Development of methods for body composition studies. Phys Med Biol 2006; 51: R203-28. [ Links ]
25. Deibert P, König D, Vitolins MZ, Landmann U, Frey I, Zahradnik HP. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre- versus postmenopausal women. Nutr J 2007; 6: 31-7. [ Links ]
26. Hernández JL, Olmos JM, González-Macías J. Metabolic syndrome, fractures and gender. Maturitas 2011; 68: 217-23. [ Links ]
27. Hadaegh F, Bozorgmanesh M, Safarkhani M, Khalili D, Azizi F. Predictability of body mass index for diabetes: affected by the presence of metabolic syndrome? BMC Public Health 2011; 11: 383. [ Links ]
28. Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 2010; 95: 5419-26. [ Links ]
![]() Correspondence:
Correspondence:
Francisco Javier Ordóñez.
Human Anatomy Department.
School of Medicine. University of Cadiz.
Pza. Fragela, s/n.
11003 Cadiz. Spain
E-mail: franciscojavier.ordonez@uca.es
Recibido: 2-VII-2013.
1.a Revisión: 20-VIII-2013.
Aceptado: 21-VIII-2013.