My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.28 n.6 Madrid Nov./Dec. 2013
https://dx.doi.org/10.3305/nh.2013.28.6.6902
ORIGINAL / Vitaminas
Prevalence of metilentetrahidrofolate reductase C677T polymorphism, consumption of vitamins B6, B9, B12 and determination of lipidic hydroperoxides in obese and normal weight Mexican population
Prevalencia del polimorfismo C677T de la metilentetrahidrofolato reductasa, consumo de las vitaminas B6, B9, B12 y determinación de hidroperóxidos lipídicos en población mexicana obesa y con peso normal
César Hernández-Guerrero1, Inés Romo-Palafox2, Mary Carmen Díaz-Gutiérrez1, Mariana Iturbe-García3, Alejandra Texcahua-Salazar3 and Ana Bertha Pérez-Lizaur1
1Departamento de Salud, Universidad Iberoamericana Ciudad de México. México.
2Estudiante de la Maestría en Nutriología Aplicada de la Universidad Iberoamericana Ciudad de México. México.
3Estudiante de la Licenciatura en Nutrición y Ciencia de los Alimentos de la Universidad Iberoamericana Ciudad de México. México.
Acknowledgements to the Universidad Iberoamericana Ciudad de México who through the Research Direction funded the project at the "5a Convocatoria Para Financiamiento de Proyectos de Investigación".
ABSTRACT
Introduction: Oxidative stress is a key factor in the development of the principal comorbidities of obesity. Methylenetetrahydrofolate reductase enzyme (MTHFR) participates in the metabolism of folate with the action of vitamins B6 and B12. The gene of MTHFR may present a single nucleotide polymorphism (SNP) at position 677 (C677T), which can promote homocysteinemia associated to the production of free radicals.
Objective: To determine the frequency of SNP C677T of the MTHFR, evaluate the consumption of vitamins B6, B9, B12 and determine the concentration of plasma lipid hydroperoxides (LOOH) in obese and control groups.
Methods: 128 Mexican mestizo according to their body mass index were classified as normal weight (Nw; n = 75) and obesity (ObeI-III; n = 53). Identification of SNP C677T of MTHFR was performed by PCR-RFLP technic. The consumption of vitamins B6, B9 and B12 was assessed by a validate survey. LOOH was determined as an indicator of peripheral oxidative stress.
Results: There was no statistical difference in the frequency of the C677T polymorphism between the TT homozygous genotype in Nw (0.19) and ObeI-III (0.25). The frequency of T allele in Nw was 0.45 and 0.51 in ObI-III group. There were no statistical differences in the consumption of vitamins B6, B9 and B12 between Nw and ObI-III groups. The LOOH showed statistical difference (p < 0.05) between Nw and ObI-III group.
Discussion: Oxidative stress is present in all grades of obesity although there were no differences in the vitamin consumption and the SNP C677T between Nw and ObeI-III groups.
Key words: Obesity. MTHFR-C677T polymorphism. Vitamins. Lipid hydroperoxide. Oxidative stress.
RESUMEN
Introducción: El estrés oxidativo es un factor clave en el inicio y el desarrollo de las comorbilidades de la obesidad. La enzima metiltetrahidrofolato reductasa (MTHFR) participa en el metabolismo del folato con la acción de las vitaminas B9 y B12. El gen MTHFR puede presentar un polimorfismo de un solo nucleótido (SNP) en la posición 677 (C677T), que puede promover homocisteinemia asociada a la producción de radicales libres.
Objetivo: Determinar la frecuencia del SNP C677T de la MTHFR, evaluar el consumo de vitaminas B6, B9, B12 y determinar la concentración de hidroperóxidos lipídicos (LOOH) en plasma en un grupo de obesos y testigo.
Métodos: Se clasificaron 128 mexicanos mestizos de acuerdo a su índice de masa corporal en normopeso (Nw; n = 75) y obesidad (ObeI-III; n = 53). Se identificó el SNP C677T de la MTHFR mediante la técnica de PCR-RFLP.
El consumo de vitaminas B6, B9 y B12 se evaluó mediante una encuesta validada. Se determinaron LOOH como un indicador de estrés oxidativo periférico.
Resultados: No hubo diferencia estadística significativa en la frecuencia del polimorfismo C677T entre homocigotos TT en Nw (0,19) y ObeI-III (0,25). La frecuencia del alelo T en Nw fue de 0,45, y 0,51 en el grupo ObeI-III. Los LOOH mostraron diferencia estadística significativa (p < 0,05) entre Nw y ObI-III.
Discusión: El estrés oxidativo está presente en todos los grados de obesidad aunque no hubo diferencia entre el consumo de vitaminas y el SNP C677T entre los grupos de Nw y ObeI-III.
Palabras clave: Obesidad. Polimorfismo MTHFR-C677T. Vitaminas. Hidroperóxidos lipídicos. Estrés oxidativo.
Abbreviations
WHO: World Health Organization
ENSA: Encuesta Nacional de Salud.
ENSANUT: Encuesta Nacional de Salud y Nutrición.
MTHFR: Mehtylenetetrahydrofolate reductase
C: Cytosine
T: Thymine Ala: Alanine
Val: Valine
SNP: Single nucleotide polymorphism
Hcy: Homocysteine
5, 10-MTHF: 5,10 methylenetetrahydrofolate
5-MTHF: 5-methylenetetrahydrofolate
TNF-α: Tumor necrosis factor α.
IL-β: Interleucine 1β.
BMI Body mass index.
PCR-RLFP: Polymerase chain reaction-restriction fragment length polymorphism
bp: Base pair.
LOOH: Lipid hydroperoxides.
BHT: Butil hydroxitolueno.
RDI: Recommended daily intake
Xi2: Chi square.
n: Number.
Nw: Normal weight.
Obe I: Obesity I.
Obe II: Obesity II.
Obe III: Obesity III.
Obe I - III: Obesity I - III.
x: Mean.
SD: Standard deviation.
me: Median
Introduction
Obesity is a multifactorial chronic disease defined as an abnormal or excessive fat accumulation in adipose tissue. Its etiology is based in a complex interaction between genetic and environmental characteristics defined by social and emotional factors. Obesity represents a high risk for the beginning and development of dyslipidemia, insulin resistance, hyperglycemia, coronary artery disease, diabetes mellitus type 2, asthma, sleep apnea, hypertension and cancer. In childhood, some important consequences of obesity include sleep apnea, orthopedic and psychosocial disorders1.
Obesity has become a worldwide public health problem, which affects all age groups, genders and ethnic populations. The World Health Organization (WHO) has declared obesity as a worldwide epidemic. Approximately 70% of adolescents grow up to be obese adults. According to the WHO in 2005, 400 million of adults were obese, and it is estimated that by 2015, 700 million adults will be obese. This epidemic is affecting developed and underdeveloped countries2.
Mexico is facing a serious health problem, the prevalence of overweight and obesity that was 62.1% in adults who were 20 years or older (including men and women) reported in the 2000 national survey (ENSA 2000)3 has increased to 71.2% according to the last national health and nutritional survey (ENSANUT 2012)4. For children, the situation is even worse, because between these two national surveys, there was an increment of 15% in children from 5 to 11 years old with overweight and obesity. The high prevalence of this phenomenon in Mexican population contributes to 33% of deaths in women and 26% in men, from chronic disease such as diabetes, cancer and cardio and cerebrovascular disorders5.
Methylenetetrahydrofolate reductase gene (MTHFR) is located in the p36.6 region of chromosome 1. This gene may present a single nucleotide polymorphism (SNP) in the exon 4 at nucleotide 677, in which a cytosine is substituted for thymine (C677T), leading to change of alanine for valine aminoacid at codon 222 (Ala222Val)6.
It is known that the presence of the polymorphism C677T in MTHFR in heterozygote carriers, accounts for a decrease in the enzymatic activity about 30%, and 70% in homozygote carriers. The abrogate activity of MTHFR reduces the concentration of folate and promotes higher serum homocysteine (Hcy) levels7.
The MTHFR enzyme plays a mayor roll in folate metabolism by catalyzing the conversion of 5,10 methylenetetrahydrofolate (5,10-MTHF) to 5-methylenetetrahydrofolate (5-MTHF), which represents the main circulating form of folate, which serves as co-substrate for the methylation of Hcy to methionine trough the methionine synthase enzyme, in the presence of vitamin B12 as co-factor.
The presence of 5MTHF as donor or the metilo group is vital to obtain methionine and maintain the consumption of Hcy in the biochemical cycle, since an increase in the concentration of Hcy in plasma, is associated to vascular damage that can initiate or accelerate thrombotic and atherogenic processes. When Hcy is free in plasma it quickly oxides generating the formation of superoxide free radical and hydrogen peroxide, which are capable of causing oxidative damage in biological cellular membranes, or initiate the peroxidation processes of lipoprotein particles in plasma8,9.
It has been reported that individuals with obesity display higher values of peripheral oxidative stress markers, due to pro-oxidant processes such as inflammatory adipokine synthesis like leptine, tumor necrosis factor-alpha (TNF-α) and interleukine-1β (IL-β) by adipocytes and macrophages. By the increases of respiratory activity required to displace a larger amount of body mass and due a lower consumption of vitamins and antioxidant molecules by this group10.
It seems that a low consumption of folate and vitamins B6 and B12 in obese people, associated with the presence of a higher prevalence of SNP C677T of MTHFR, can be factors that account low availability of the substrate and cofactors necessary for the generation of 5-MTHF.
The present study's goal was to identify the frequency of the polymorphism C677T of the MTHFR in Mexican mestizo population of the central plateau who had normal weight, overweight and various degrees of obesity. To evaluate the consumption of folate, vitamin B6 and B12, and to determine the concentration of lipid hydroperoxides in serum as an oxidative stress marker.
Material and methods
Study population
The work included 128 participants who attended the Nutrition Clinic at Universidad Iberoamericana in Mexico City. The participants were Mexican mestizo from the central plateau who's family background was assessed. All subjects reported that at least their parents and grandparents were born in Mexico. All participants were 18 years old or older and denied any thyroid, autoimmune, allergic or eating disorder, without smoking or alcoholism history or significant weight variation in the last six months; women were not pregnant o lactating.
Body mass index (BMI) was determined with the evaluation of height using a Seca® model 240 (Vogel &Halke GMBH & Co., Germany) stadimeter, with a precision of ±2 mm. For the weight evaluation participants were barefoot with light clothing with a body composition by bioimpedance Tanita® model TBF-300A (Corp. of America Inc., Arlington Heights, Illinois), with a precision of ±0.1 kg and a capacity of 200kg. Subjects were classified into two groups according to their BMI (BMI; kg/m2); normal weight group (BMI 18.5-24.9), which included 53 participants, and obesity group (BMI ≥ 30) with 80 patients.
The size of the sample was estimated based on the grade of lipoperoxidation in plasma, according to the hypothesis test to differentiate between two proportions with a test power of 10% and a level of a of 0.05. In order to do this, it was assumed that the individuals with normal weight would present 20% less lipidic hydroperoxidation in plasma in comparison with patients with obesity, which generated a total of 46 participants per group. The number of patients in the obesity group was augmented in order to have a larger number of participants when the stratification was made, which generated a distribution according to BMI of 26 individuals with obesity type I (BMI 3034.9), 18 for type II (BMI 35-39.9) and 31 for type III (BMI > 40).
All participants were previously informed of the characteristics and the scope of the study, and written informed consent was obtained from each participant, and ethical principals of Helsinki declaration were followed. Thus, the project was examined and approved by the ethic and scientific committee of the University.
Blood samples collection and DNA extraction
Peripheral fasting blood samples were taken during their evaluation. Blood samples were collected in 7-mL heparin tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA), and taken to the laboratory immediately for DNA extraction. Isolation of DNA was performed from whole blood (100 μL) using DNAzol (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer's instructions.
Determination of MTHFR C677T polymorphism
Identification of cytosine or thymine nucleotide in the +677 position of MTHFR was performed using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RLFP) technique according to Skibola et al11 using the following primers: forward 5 -TGA AGG AGA AGG TGT CTG CGG GA-3 and reverse 5 - AGG ACG GTG CGG TGA GAG TG-3 .
Protocol included 35 cycles (30 seconds) at 94oC for denaturation, 60o C for annealing and 72o C for elongation. The amplified product (10 μL) was digested with Hinf I (4 U) restriction enzyme (Roche Molecular Biochem, Mannheim, Germany) during 24 h at 37o C and analyzed by electrophoresis on polyacrylamide gels (8%) to improve the DNA-bands resolution. The DNA-bands in the electrophoresis gel were identified by ethidium bromide using an image analyzer. Wild type (677CC) produced a single band of 198 bp and homozygous mutant (677TT) produced 175-, and 23-bp fragments.
Determination of frequency of vitamin B6, B9 and B12 consumption
To establish the antioxidant consumption in both groups we applied a dietary assessment food frequency questionnaire for antioxidants and retinol, validated by the National Institute of Mexican Public Health. We used the complete questionnaire without modifications. In order to make it easier for the patient to understand the questionnaire we used food comparative models, measures and portions. It was emphasized that the answers were only for the patient, and not the rest of the family. The results were analyzed with the SNUT program, which generates values of the nutriments or molecules in units of mass or international units12.
Determination of lipid hydroperoxides in peripheral blood
Total lipid hydroperoxides (LOOH) were determined using the FOX method described by Zhen-Yue13.This method is based in the oxidation of ferrous ion to ferric ion by the LOOH in acid conditions. The ferric ions produced during the reaction react with the orange xilenol reactive generating a chromophore with an extinction coefficient of 560 nm. For the determination, 300 μL of the sample and 2.7 ml of FOX reagent previously elaborated (880 mg of BHT in 900 ml of pure ethanol and 76 mg of xylenol orange and 98 mg of ammonium sulfate in 100 ml of sulfuric acid 250 mM), were incubated for 30 minutes at room temperature and controlled light conditions. The samples were then centrifuged at 400 x g for 15 minutes and the supernatant was read in a spectrophotometer UV/Vis Lambda 45 (PerkinElmer Instruments, California USA) at 560 nm. A standard curve of hydrogen peroxide in concentrations from 0 to 100 M was used for calculations.
Statistical analysis
The method of descriptive statistic was used to analyze the results of the variables obtained from the participants of the study. The difference in the consumption of vitamins between the obese group and the normal weight group was evaluated using ANOVA from Kruskal-Wallis, applying the Dunn test as pos hoc analysis. The comparison between before mentioned groups in the lipid hidroperoxidation was evaluated by Mann-Witney U study. The genetic frequency of the polymorphism, the alleles and the number of participants under the RDI of vitamins B6, B9 and B12 were analyzed using Xi2 test. A value of p less o equal to 0.05 was accepted as a statistical difference. Statistical analyses were performed using the SigmaStat v.3.5 software (Systat Software Inc., San Jose, CA, USA).
Results
Study population
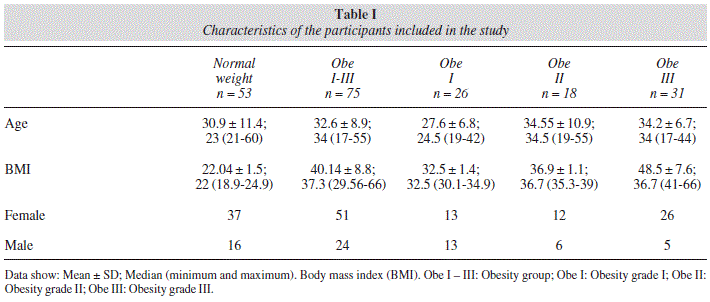
The study included 128 adult participants between 18 and 60 years old, from which 68.75% (n = 88) were women and 31.25% (n = 40) were men. The control group was formed by 53 participants and the obesity group by 75 participants, who were stratified into three groups depending on the grade of obesity, which included 26, 18 and 31 individuals in grade I, II and III, respectively. The age and sex distribution for each group are described in table I. There was no statistical difference in the age of the participants from the obesity group when they were compared intragroup, or against the control group.
Frequency of the C677T polymorphism of MTHFR
The frequency of the C677T polymorphism in subjects with normal weight showed a value of 0.28 for the CC homozygous genotype, while the obesity group the value was 0.24. There was no statistical difference when comparing the frequencies between groups, not even when analyzing the frequency of the mutated genotype by carriers (heterozygote plus homozygote) between people with normal weight (0.72) and obesity (0.76). There was no statistical difference when stratifying in grades of obesity and comparing them with the normal weight group. Table II shows the frequencies of the polymorphism C677T and the alleles C and T observed in the study and control group. A representative image of identification of wild type, heterozygote and homozygote mutant carriers is shown in the figure 1. The MTHFR genotype distribution was compatible with the Hardy-Weinberger equilibrium (X2 = 0.0098; p = 0.92).
Frequency of consumption of vitamin B6, B9 and B12
The frequency of consumption of vitamin B6 by each obesity group and by the control group is presented in figure 2A. Statistical difference (p < 0.05) was observed only between the normal weight group (x = 1.67 +/- 1.13; me = 1.47 ng/mL) and the obesity III group (x = 2.26 +/- 1.31; me = 1.95 ng/mL).
For vitamin B9 the results of the frequency of consumption are shown in figure 2B. The comparison between groups only showed statistical difference (p < 0.05) between normal weight group (x = 1140 +/- 1217; me = 1020 ng/mL) and the obesity grade I (x = 451 +/-429; me = 337ng/mL). The frequencies of consumption of vitamin B12 by the study and control groups are shown in figure 3A. No statistical difference was observed when comparing the values obtained between the group with normal weight and with obesity, when participants were analyzed in the same group or when they were stratified by grades of obesity.
On the other hand, for the three vitamins analyzed in the present study all the participants included in the groups were under the RDI. Only for the folate there were statistical differences between the obese grades I and II, in comparison with the normal weight group, respectively (Table III).
Lipid hydroperoxidation in peripheral blood
The results of the lipid peroxidation in the peripheral compartment of the study groups and control group are shown in figure 3B. We observed a statistical difference (p < 0.05) between the group with normal weight (x = 4.78 +/-3.22 μM; me= 4.65 μM) and the obesity group (x = 11.96 +/-3.52 μM; me= 11.42 μM). When stratifying the participants of the study group in grades of obesity, we observed a statistical difference between the normal weight group and the three obesity grades (p < 0.05).
Discussion
The results obtained in the present study did not show statistical difference in the prevalence of the C677T polymorphism of the MTHFR between individuals with normal weight and individuals with obesity. The frequencies observed with the genotypes CC, CT and TT, and the C and T alleles in individuals with normal weight were very similar to the values reported by Davalos in 200014 in Mexican mestizo population. The frequency (0.19) of the TT genotype observed in the present study is nearly double of the value reported by Mutchinic (0.34) in 199915 and by Gueant-Rodriguez (0.35) in 200616, which analyzed the same type of population. The possible explanation of before mentioned differences can be attributed to the high variation that the polymorphism displays worldwide, and particularly in our country. Mutchinic himself reported a frequency of the TT genotype of 0.22 in Mexican mestizo population from the north, and a value of 0.47 in the same population from the center of the country15. On the other hand, there are no previous reports about the frequency of this polymorphism in persons that present obesity in our country; therefore it is necessary to perform more studies that reinforce or not our findings.
Worldwide, in 2007 Terruzzi17 found an association between Italian subjects with obesity and the presence of the A1298C polymorphism of the MTHFR, but not with the C677T polymorphism. Settin in 200918 and Riu-Xing in 201219 did not identify an association between the polymorphism herein studied and persons with overweight and obesity from Arabia and China, respectively. Lui in 200820 studied 8 polymorphisms of the MTHFR in 1873 subjects of the USA population of European ancestry, and found association between the lean body mass showed by the participants, and three polymorphisms rs3737964, rs2066470 and rs4846048) of the MTHFR enzyme (none of which studied herein). Previous reports highlight the wide distribution that this polymorphism shows in the world population, and its possible association with obesity and its main comorbidities, since in the Hindu21 and Egyptian population22 there have identified an association between the C677T polymorphism and the presence of diabetes type 2.
The MTHFR is a key enzyme in the metabolism of folate which acts as a substrate for the methylation of Hcy and its conversion to methionine. The presence of mutated genotype of the MTHFR in the heterozygous form (CT), accounts a reduction in the enzymatic activity around 30%, and this value can rise up to 70% if the genotype is homozygous (TT). Even though in our study we did not identify a different prevalence of the C677T polymorphism in persons with obesity in comparison with the control group, the values of frequency by carriers (homozygous plus heterozygous) are high in the normal weight group (0.72) and in the obesity group (0.76). The reduction in the biologic activity of the MTHFR when the polymorphism is present plus the availability of vitamins B6, B9 and B12 in the organism, can generate hyperhomocysteinemia and this promotes the appearance of oxidative stress, as well as a risk factor identified for the development of atherosclerotic and cardiovascular diseases23, 24.
In our study, we evaluated the frequency of consumption of vitamins B6, B9 and B12 in the participants, and we did not identify statistical differences when comparing the results between the normal weight and obese group. However when the obese group was stratified in grades of the disease, the group of grade I presents fewer consumption of folate versus the control group, and the group III of obesity consumes more vitamin B6 in comparison with the control group, both comparisons with statistical differences.
The few intake of folate showed by the obese grade I is in line with previous reports, in which a decreased concentration of folate was observed in the plasma of obese people in comparison with normal weight controls25,26. Or when the consumption of folate was evaluated as in the present manuscript and a lesser intake of vitamin was identified27.
However, Gil in the 201128 assayed the concentration of vitamins B12 and folate in the plasma of 497 morbid obese people with metabolic syndrome, and reported that all the participants showed concentrations of both vitamins within the normal value. These data reinforces the need to address the study taking into account the genetic, environmental and nutritional characteristics involved in the etiology of obesity in a particular group or population, since specific conditions present in a population could not be present in other population with similar characteristics, and vice versa. Certainly, knowing this information could improve the clinical and nutritional maneuvers applied in the target subjects, aimed to reducing the obesogenic phenomena.
For the vitamin B6 the higher consumption shown by morbid obese people of this molecule, could be associated with the higher food consumption showed by this group, due to the fact that this vitamin is present in almost all food group29.
On the other hand, for the folate we observed a decreased proportion of people (statistically different) with obesity who consumes this vitamin below the RDI in comparison with normal weight people. Also, this vitamin is consumed in a lesser quantities compared to the RDI, considering that around 30% and 40% of the participants from control and obese groups are under the RDI, respectively.
In the present manuscript we included adults with normal weight and obesity from 18 to 60 years old, however the results before mentioned are consistent with the manuscript reported by Al-Taham, who in a wide revision of the literature (1980-2004) about the intake of vitamin B-complex by European adolescents, the author reported that folate is only the vitamin who shows a consumption under the RDI by the group studied30.
On the other side, we did not find statistical differences in the number of participants that consume the vitamins B6 and B12 under the RDI when the control and obese groups were compared, however both groups were under the RDI in approximately 20% for the vitamin B6 and 10% for vitamin B12.
We think that the combination of a high prevalence of the mutated genotype of the MTFR in the studied population, plus in general the lesser consumption of vitamins B6, B9 and B12 by the individuals with obesity, can be part of the factors involved in the greater concentration of lipidic peroxides identified in plasma, since it has been describes that the low consumption of vitamin B6, B9 and B12 in the diet relates with hyperhomocysteinemia which is capable of promoting the generation of free radicals and oxidative stress. In our study we observed a greater concentration of lipidic peroxides in individuals with obesity in comparison with the control group. Although we have the limitation of not establishing the concentration of homocystein, it is clear that the presence of obesity is associated with a higher status of oxidative stress, since during the stratification in grades of obesity a statistical difference was observed in all groups when comparing them to the control group.
The people with obesity have physiologic conditions that promote the generation of free radicals, which if they not adequately neutralized are capable of generating oxidative stress. This condition has been pointed as an outstanding phenomenon in the appearance and development of the main comorbidities of obesity.
Increase the knowledge of the specific characteristics related to appearance of oxidative stress in a specific group of persons with obesity is an essential necessity, to improve the clinical and nutritional maneuvers directed to decrease or avoid its appearance.
Acknowledgements
To the Universidad Iberoamericana Ciudad de México who through the Research Direction funded the project at the "5a Convocatoria Para Financiamiento de Proyectos de Investigación". To the team of MTHFR polymorphism C677T and vitamin consumption in obesity the Nutrition Clinic and Laboratory of Research of the Health Department of the University, by their enthusiastic participation. To all the participants included in the study for their valuable collaboration.
References
1. Orzano A, Scott J. Diagnosis and treatment of obesity in adults: an applied evidence-based review. J Am Board Fam Pract 2004; 17: 359-69. [ Links ]
2. World Heath Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation. WHO Technical Report Series. Geneva; 2000. [ Links ]
3. Olaiz G, Rojas R, Barquera S, Shamah T, Aguilar C, Cravioto P, López P, Hernández M, Tapia R, Sepúlveda J. Encuesta Nacional de Salud 2000. Tomo 2. Cuernavaca, México: Instituto Nacional de Salud Pública. [ Links ]
4. Gutierrez JP, Rivera-Dommarco J, Shamah-Levy T, Villal-pando-Hernandez S, Franco A, Cuevas-Nasu L, Romero-Martinez M, Hernandez-Avila M. Encuesta Nacional de Salud y Nutricion 2012. Resultados Nacionales. Cuernavaca, México: Instituto Nacional de Salud Publica (MX), 2012. [ Links ]
5. Secretaría de Salud. Dirección General de Información en Salud. Causas de muerte según códigos de la Clasificación Estadística Internacional de Enfermedades y Problemas Relacionados con la Salud. Décima Revisión (CIE- N). Consejo Nacional de Población. México, 2006. [ Links ]
6. Kennedy DA, Stern SJ, Matik I, Moretti ME, Sarkar M, Adams-Webber T, et al. Folate Intake, MTHFR Polymorphisms, and the Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis. J Cancer Epidemiol 2012. pp. 1-24. [ Links ]
7. Cardona H, Cardona-Maya W, Gómez JG, Castañeda S, Gómez JM, Bedoya G, et al. Relación entre los polimorfismos de la metilen-tetrahidrofotato-reductasa y los niveles de homo-cisteína en mujeres con pérdida gestacional recurrente: perspectiva desde la nutrigenética. Nutr Hosp 2008; 23 (3): 277-82. [ Links ]
8. Sánchez C, Planells E, Aranda P, Pérez de la Cruz A, Asensio C, Mataix J, et al. Vitaminas B y homocisteína en la insuficiencia renal crónica. Nutr Hosp 2007; 22 (6): 661-71. [ Links ]
9. Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 1996; 98: 5-7. [ Links ]
10. Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes 2006; 30: 400-18. [ Links ]
11. Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cartwright RA, Morgan G. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA 1999; 26 (22): 12810-5. [ Links ]
12. Hernández-Avila M, Romieu I, Parra S, Hernández-Avila J, Madrigal H, Willet W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City*. Instituto Nacional de Salud Pública 1998; 40 (2): 133-40. [ Links ]
13. Jiang Z-Y, Hunt JV, Wolff SP. Ferrous Ion Oxidation in the Presence of Xylenol Orange for Detection of Lipid Hydroperoxide in Low Density Lipoprotein. Anal Biochem 1992; 202: 384-9. [ Links ]
14. Dávalos I-P, Olivares N, Castillo M-T, Cantú J-M, Ibarra B, Sandoval L, et. al. The C677T polymorphism of the methyl-enetetrahydrofolate reductase gene in Mexican mestizo neuraltube defect parents, control mestizo and native populations. Ann. Genet2000; 43: 89-92. [ Links ]
15. Mutchinichk OM, López MA, Luna L, Waxman J, Babinsky VE. High Prevalence of the Thermolabile Methylenetetrahydrofolate Reductase Variant in Mexico: A Country with a Very High Prevalence of Neural Tube Defects. Mol Genet Metab 1999; 68: 461-7. [ Links ]
16. Guéant-Rodríguez RM, Guéant JL, Debard R, Thirion S, Hong LX, Bronowicki JP, et. al. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. Am J Clin Nutr 2006; 83: 701-7. [ Links ]
17. Terruzzi I, Senesi P, Fermo I, Lattuada G, Luzi L. Are genetic variants of the methyl group metabolism enzymes risk factors predisposing to obesity? J Endocrinol Invest 2007; 30 (9): 747-53. [ Links ]
18. Settin AA, Algasham A, Dowaidar M, Ismail H. Methylene tetrahydrofolate reductase and angiotensin converting enzyme gene polymorphisms related to overweight/obesity among Saudi subjects from Qassim Region. Dis Markers 2009; 27: 97-102. [ Links ]
19. Yin R-X, Wu D-F, Miao L, Htet Htet Aung L, Cao X-L, Yan T-T, et al. Several genetic polymorphisms interact with overweight/obesity to influence serum lipid levels. Cardiovasc Diabetol 2012; 11: 123. [ Links ]
20. Liu X, Zhao L-J, Liu Y-J, Xiong D-H, Recker RR, Deng H-W. The MTHFR gene polymorphism is associated with lean body mass but not fat body mass. Hum Genet 2008; 123: 189-96. [ Links ]
21. Chauhan G, Kaur I, Tabassum R, Om Prakash Dwivedi, Ghosh S, Tandon N, et al. F Variants of Homocysteine Metabolism Pathway Genes and Risk of Type 2 Diabetes and Related Traits in Indians. Exp Diabetes Res 2012. pp. 1-7. [ Links ]
22. AbdRaboh NR, Badr S, Ali S. Prevalence of methylenetetrahy-drofolate reductase C677T and A1298C polymorphisms in Egyptian patients with type 2 diabetes mellitus. Egypt J Med Hum Genet 2013; 14: 87-93. [ Links ]
23. Varela-Moreiras G, Escudero, JM, Alonso-Aperte E. Homocisteína, vitaminas relacionadas y estilos de vida en personas de edad avanzada: estudio SÉNECA. Nutr Hosp 2007; 22 (3): 363-70. [ Links ]
24. Bayo MP, Olivares López MJ, Osuna Ortega A, Peinado A, Quesada Granados JJ, López García de la Serrana H, et al. Resistencia de la hiperhomocisteinemia del paciente renal al tratamiento con dosis suprafisiológicas de ácido fólico parenteral. Nutr Hosp 2008; 23 (3): 268-76. [ Links ]
25. Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol 2004; 19 (11): 1029-36. [ Links ]
26. Hirsch S, Poniachick J, Avendaño M, Csendes A, Burdiles P, Smok G, et al. Serum folate and homocysteine levels in obese females with non-alcoholic fatty liver. Nutrition 2005; 21 (2): 137-41. [ Links ]
27. Kimi Uehara S, Rosa G. Association of uricemia with biochemical and dietary factors in human adults with metabolic syndrome genotyped to C677T polymorphism in the methylenetetrahydrofolate reductase gene. Nutr Hosp 2011; 26 (2): 298-303. [ Links ]
28. Ruano Gil M, Teruel S, Aguirregoicoa Garcia E, Criado Gomez L, Duque Lopez Y, Garcia-Blanch G. Nutrición, síndrome metabólico y obesidad mórbida. Nutr Hosp 2011; 26 (4): 759-64. [ Links ]
29. Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, Gonzalez-Gross M. Vitamin B6 status, deficiency and its consequences - an overview. Nutr Hosp 2007; 22 (1): 7-24. [ Links ]
30. Al-Tahan J, González-Gross M, Pietrzik K. B-Vitamin status and intake in European adolescents. A review of the literature. Nutr Hosp 2006; 21 (4): 452-65. [ Links ]
![]() Correspondence:
Correspondence:
César Hernández Guerrero.
Departamento de Salud.
Universidad Iberoamericana Ciudad de México. México.
Prolongación Paseo de la Reforma, 880.
01219 Lomas de Santa Fe. México. Distrito Federal.
E-mail: cesar.hernandez@uia.mx
Recibido: 24-IV-2013.
1.a Revisión: 14-VIII-2013.
Aceptado: 20-VIII-2013.