Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.29 no.2 Madrid feb. 2014
https://dx.doi.org/10.3305/nh.2014.29.2.7076
REVISIÓN
Intestinal permeability measurements: general aspects and possible pitfalls
Mediciones de permeabilidad intestinal: aspectos generales y posibles riesgos
Tatiana Fiche Salles Teixeira1, Ana Paula Boroni Moreira1, Nilian Carla Silva Souza2, Rafael Frias3 and Maria do Carmo Gouveia Peluzio1
1Department of Nutrition and Health. Federal University of Viçosa. Brazil.
2National Institute of Cancer. Rio de Janeiro. Brazil.
3Central Animal Laboratory. University of Turku. Finland.
ABSTRACT
Introduction: Disturbances of the gut barrier function have been related to a variety of diseases, including intestinal and extra-intestinal diseases. The intestinal permeability tests are considered useful tools for evaluating disease severity and to follow-up patients after a therapeutic intervention and indirectly assess barrier function.
Objective: The aims of this review were to highlight the possible factors underlying higher intestinal permeability and the clinical conditions that have been associated with this in different age range; and also provide some insight into methodological aspects.
Results and discussion: Abnormal regulation of tight junction function is the main cause of altered intestinal barrier. The impaired barrier function results in higher permeation rates of administered probes through the intestinal mucosa. Lactulose and mannitol are one of the most commonly used probes. The innocuousness and easiness of intestinal permeability tests can be explored to expand the knowledge about the clinical situations in which intestinal barrier dysfunction can be an important feature. Many factors may influence the results of the test. Researchers and healthcare professionals should try to circumvent the possible pitfalls of the intestinal permeability tests to produce consistent evidences. The use of others markers of intestinal physiology may also contribute to understand the role of barrier function in different diseases.
Key words: Intestinal permeability. Gut barrier. Lactulose. Mannitol.
RESUMEN
Introducción: Alteraciones funcionales de la barrera intestinal se han relacionado con una variedad de enfermedades intestinales y también con enfermedades no intestinales. Las pruebas de permeabilidad intestinal son consideradas herramientas útiles para evaluar la gravedad de la enfermedad para el posterior seguimiento de los pacientes después de una intervención terapéutica.
Objetivo: El objeto de esta revisión ha sido destacar los posibles factores que pueden estar asociados a una mayor permeabilidad intestinal y revisar condiciones clínicas que han sido asociadas en individuos de diferentes edades. También revisar ciertos aspectos metodológicos de las pruebas de permeabilidad intestinal.
Resultados y discusión: Las uniones estrechas entre los enterocitos son las principales estructuras encargadas de la regulación de la barrera intestinal. Una alteración de éstas, resulta en una deficiencia en la permeabilidad intestinal y una mayor penetración de las sustancias marcadoras de permeabilidad intestinal. La lactulosa y el manitol son las sustancias marcadoras más utilizadas. La inocuidad y facilidad de los test de permeabilidad han sido de ayuda para explorar y ampliar el conocimiento de muchas condiciones clínicas en las que la disfunción de la barrera intestinal ha sido un sello distintivo. Muchos factores pueden influir en los resultados de los test de permeabilidad. Sin embargo, los investigadores y los clínicos han de tratar de eludir los posibles inconvenientes de las pruebas de permeabilidad intestinal para poder producir evidencias más consistentes. El uso de otras sustancias marcadoras de la fisiología intestinal también puede contribuir a comprender mejor el papel de la barrera intestinal en diferentes enfermedades.
Palabras clave: Permeabilidad intestinal. Barrera intestinal. Lactulosa. Manitol. Fisiología intestinal.
Abbreviations
BMI: body mass index.
IP: intestinal permeability.
GFR: glomerular filtration rate.
L/M: lactulose/mannitol ratio.
TJ: tight junctions.
Introduction
The gastrointestinal tract has the complex task of absorbing nutrients while excluding the uptake of dietary antigens, luminal microbes and their products. The intestinal mucosa exhibit a selectively permeable barrier property, which supports this task. The histological organization of the gastrointestinal tract mucosa and the interaction between cellular (polarized epithelial cell membrane, tight junctions (TJ), lymphocytes) and extracellular components (mucin, unstirred layer of fluid)1-4 are essential for the gut barrier function. Homeostasis of gut barrier function is critical for the ability of gastrointestinal tract to articulate aggressive reactions against enteric microbes while developing oral tolerance for food antigens and commensal bacteria5.
Disturbances of the gut barrier function have been related to a variety of clinical conditions in different age range (Tables I and II)2,6. The investigation of gut barrier dysfunction and other intestinal abnormalities (such as polyps, tumors) can be done through methods such as collection of a biopsy sample using surgical and/or endoscopic procedures. However, these procedures are invasive, often inconvenient to the patient and usually imply high healthcare costs7. This has led to the development of alternative methods to assess gut barrier function while preventing patients from undergoing such kind of invasive methods.
Intestinal permeability (IP) tests represent one alternative method. The concept of intestinal epithelial batrier function is tightly related to the concept of permeability, which is the property of the membrane to allow non-mediated solute diffusion8-9. When the barrier is intact, the permeability of substances is highly selective and controlled. Disturbances in gut barrier function can affect the control of permeating substances9,10. Based on these principles the oral administration of specific probes has been commonly used to indirectly assess gut barrier dysfunction and measure IP. These probes are subsequently quantified in blood or more frequently in urine11. In a simplistic way, injuries in the intestinal mucosa can impair its barrier function. The impaired barrier function results in higher permeation rate of probes and intact proteins through the intestinal mucosa12,13.
Intestinal permeability tests are not widely used in clinical practice. Their use has been usually restricted for scientific purposes. However, evaluation of IP can be a useful tool in screening for small intestinal disease, in assessing the response in the follow-up period after a therapeutic intervention and in predicting the prognosis, especially in celiac disease14,15. The majority of probes used have been shown to be non-toxic to patients and relatively easy to quantify. These characteristics can be explored by medical professionals to expand the knowledge about the clinical situations in which intestinal barrier dysfunction can be an important feature.
In this context, the aims of this review were to highlight the possible factors underlying higher IP and the clinical conditions that have been associated with this in different age range; and also provide some insight into methodological aspects to be considered in future studies.
Methods
Medline/Pubmed, Scielo and Lilacs were used to search for articles accomplishing the following terms (alone or associated): intestinal or gut permeability, intestinal or gut barrier, lactulose, mannitol, tight junctions. Review and original articles were selected and read critically.
Factors underlying increased intestinal permeability
The intestinal epithelium is a single layer of columnar epithelial cells that separates the intestinal lumen from the underlying lamina propria. It is believed that there are two routes for substances permeation through the intestinal epithelial cells: transcellular (across the cells, both by active and passive processes), and paracellular (between adjacent cells, by a passive process)16,17. The epithelial cells are tightly bound together by intercellular junctional complexes. They are formed by TJ, gap junctions, adherens junctions and desmosomes. The space between cells is called paracellular space. The permeability of molecules through this space is under control of the junctional complexes, which are crucial for the integrity of the epithelial barrier17.
Tight junctions are complex structures comprising over 50 types of proteins (claudin, occludin, zonulin, junctional adhesion molecules). They form a continuous, circumferential seal around cells through the interaction with the perijunctional actomyosin ring of epithelial cells17. It has been observed that TJ have a central role in processes that regulate epithelial proliferation and differentiation18.
Regulation of the assembly, disassembly and maintenance of TJ structure is influenced by various physiological and pathological stimuli. The knowledge of how TJ are modified in response to signals that alter their functional properties is of great importance in the context of diseases associated with altered IP16,19-21. Experimental studies using animal and cell culture models or human studies have shown that deregulated TJ are the main cause of altered intestinal barrier. This alteration can be induced by endogenous and exogenous factors (Table III).
Recently, it has been demonstrated that increased IP can occur due to discontinuities in the epithelial cell layer in the gut. These discontinuities are called gaps and have been identified in the mouse and humans. They are formed when epithelial cells leave the epithelium. These gaps have the diameter of an epithelial cell and are devoid of cellular contents, but filled with an unknown substance that maintains local barrier function. The rate at which cells leave may have implications for the permeability of the epithelium as a unit. The processes that control the rate of cell egress have not been well defined. This mechanism of increased permeability may be important in human diseases22,23.
As summarized by Teshima and Meddings22 "simply measuring an increase in permeability provides no information to the physician about the mechanisms underlying the abnormality. However, an understanding of these mechanisms may prove valuable in designing interventions". Thus the main causes of increased IP that should guide the development of efficacious intervention are: genetic alterations of TJ proteins, abnormal microbiota, abnormal regulation of TJ function (increased zonulin release), mucosal inflammation and abnormal epithelial dynamics22.
General aspects of intestinal permeability tests
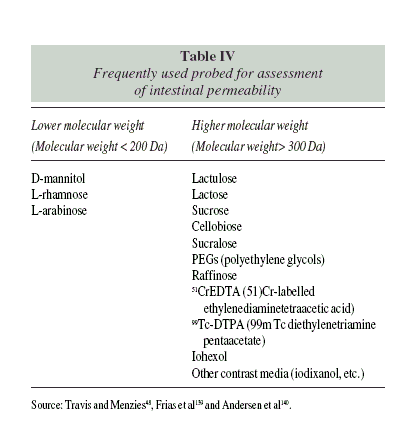
Intestinal permeability tests are based on probes of different molecular weight, which determines the route of permeation (Table IV). Smaller molecules usually permeate through membrane pores. They are expected to be present in urine in higher proportion (10 to 30% of an orally ingested dose)24. Less than 1% of higher molecular weight molecules are expected to be recovered in urine after an oral dose25. These molecules need to cross the barrier through the paracellular route, which is more tightly regulated by protein complexes.
The choice of probes depends on the intention of what part of intestine is meant to be assessed. Usually, recovery of sucrose in the urine reflects gastroduodenal permeability26, since sucrose is rapidly hydrolyzed by sucrase-isomaltase upon entering the duodenum and reflects absorption only in the most proximal portion of the gut27. Lactulose and mannitol, which are one of the most commonly used probes, are destroyed in the caecum and provide information regarding the small intestinal epithelium16. Sucralose is an artificial sweetener with similar molecular weight of lactulose and is resistant to bacterial fermentation28. It spends most of a 24 hour exposure period in the large intestine16.Therefore, sucralose has been suggested as better suitable sugar for whole gut permeability assessment29.
An inconvenience of IP tests is the prolonged period of urine collection, usually 5 to 6 hours. The introduction of sucralose has been suggested as better suitable sugar troduction of sucralose into permeability measurements might extend the test period up to 24 hours, making it less convenient in clinical practice. McOmber and co-workers recommend reexamining the usual 5 to 6 hours collection times to compare healthy individuals to those with abnormal permeability, because this period of time might not include the point of maximal urinary recovery. They studied the recovery of sucrose, lactulose, mannitol and sucralose over a 24 hours period in healthy adults and children30. It was suggested that by using different collection periods greater differences may be seen between groups with less interindividual variation: 4 to 6 hours for sucrose, 13 to 15 hours for lactulose, mannitol and sucralose. If sucralose/lactulose ratio is to be measured, collection time might be extended to 16 to 18 hours30. However, Akram and co-workers31 have compared different urine times collection and their results suggest that the use of Lactulose/Mannitol (L/M) ratio to assess IP could be simplified by shortening the time of urine collection31. The reduction of the time can also be achieved by measuring the probes in blood 60-90 min post-ingestion of solution32,33. More studies are needed to confirm that prolonged time collection is not needed.
The calculation of the ratio between sugar probes used (such as L/M) is considered a good marker of small intestinal permeation9. It is meant to circumvent confounding factors as interindividual variation of gastric emptying, intestinal transit and transport, blood distribution and renal clearance34.
In general, the integrity of intestinal barrier function is dependent on healthy epithelial cells and on the proper functioning of the paracellular route9. Theoretically, an increase in the sugar probes ratio -for example L/M ratio- would indicate altered IP. This alteration may reflect a decrease in smaller probes (e.g. mannitol) absorption and/or an increase in the absorption of higher weight probes (e.g. lactulose). Decreased small weight probes absorption can be the result of a diminished absorptive area. Increased permeation of higher weight probes may be due to a facilitated diffusion of this marker into the crypt region as a consequence of decreased villous height or TJ loosening35.
The results of IP tests are usually expressed as percentage of excretion of probes (Table V). Other units can be also found (mg/mL, mmol/L, mg) 11,31,32,36,37.
Possible pitfalls in intestinal permeability tests
Many factors may influence the results of the test, as shown in table III. Thus, possible pitfalls for the IP tests may be circumvent by researchers or healthcare professionals when considering some details.
Previous orientation of individuals to avoid -few days before the test- the use of non-steroidal inflammatory drug38,39, acute alcohol ingestion32,40,41, psychological and physical stressful situations42-44 should be given as part of the protocol. Considering that some genetic background may exert negative influence on barrier function, family history of inflammatory bowel diseases should be considered before inclusion of patients in a study. Regarding the personal medical history some clinical factors influencing IP such as food allergy, human immunodeficiency virus, diabetes, starvation, iron deficiency, diarrhea, viral gastroenteritis, smoking45-48 should be an exclusion criteria, except if this is the topic under investigation. Additionally, search for evidence of endoparasite infection in the stools should be ideally performed before inclusion of individuals in the study49.
Usually, all tests are performed under overnight fast (8 to 10 hours). Few authors mention the instruction of individuals to follow a diet free of the sugars used as probes in the test at least 24 hours before it13,32,50. Lactulose, mannitol and sucralose are commonly used in IP tests and can be present in some common foods (Table VI). An important issue mentioned in some protocols to circumvent the possible influence of the intake of the same sugars that will be used in the IP test is the collection of a urine sample before the administration of the sugar probes. The amount of sugar quantified in this sample should be subtracted from the results in the urine collected after the ingestion of the probes13,28,33,50. Avoidance of some foods should be also advised when they contain other sugars that can imply in methodological difficulties to properly quantify the probes. Farhadi and co-workers recommend subjects to avoid consumption of dairy products on the previous day of the test since lactose peak tend to overlap that of lactulose51. During the IP test- in some studies it is mentioned that subjects are encouraged to drink water and/or to have a snack after 1 to 2 hours of probes administration11-13,37. It is not clear if this can affect the results. However- an important detail of this practice is to standardize the type of food and the volume of liquid offered to all individuals. Mattioli and co-workers52 found that the L/M ratio was significantly lower in subjects that excreted more than 500 mL of urine. The greater urine volume was associated with a higher mannitol recovery. Thus- they emphasized that urine volume may influence urinary excretion of sugar probes and intake of liquids should be carefully monitored before and during the test52.
It is noteworthy that Camilleri and co-workers question the concept that lactulose and mannitol in urine collected between 0 to 6 hours reflect small intestine permeability. They have investigated the administration of these probes (radiolabelled) in a liquid formulation or in a delayed-release methacrylatecoated capsule. It was showed that after 2 h of liquid formulation intake around 50% of the probes was in the colon- suggesting that sugars may not be absorbed exclusively in the small intestine. Thus- they suggest that the interpretation of the 0 to 6 hours differential two sugar urine excretion as an exclusive marker of small IP should be done cautiously24.
Osmolarity of test solutions should be mentioned in every study- since stress induced by high osmolarity can stimulate intestinal motility53 and change the rate of sugars permeation8. The amount of sugar administered and the volume of solutions vary between studies (see Tables I and II). In addition, the volume of solution administered is fixed for all subjects. Exception is observed in some studies with children, that use body weight to calculate the volume of solution to be administered individually50,54. This might have been proposed based on pharmacokinetics studies. At least for children, drugs dosages are based on body weight or body surface area since body size, proportion, organ development and function affect the pharmacokinetic behavior of many drugs55. It should be further discussed the possibility of using weight to calculate the volume of solution to be administered also to adult subjects. The body weight or body mass index (BMI) of subjects included in the majority of studies is not mentioned. Could this make any difference for the interpretation of IP results?
A higher BMI is associated with higher filtration fraction. This means that there is a higher glomerular filtration rate (GFR) relative to effective renal plasma flow, suggesting an altered afferent/efferent balance and higher glomerular pressure56. In obese subjects, the values for GFR exceeded by 61% the values for GFR of the control group and by 32% the value of renal plasma flow, suggestive of glomerular hyperfiltration. The obesity-related glomerular hyperfiltration ameliorates after weight loss57. It is a possible pitfall when subjects with excess of weight are included in studies: could a higher amount of excreted sugar be a consequence of higher intestinal absorption (due to higher IP) or of a higher glomerular hyperfiltration? This has not been investigated in humans. Whenever overweight and obese subjects are submitted to IP test it should be investigated if they present normal renal function (impaired renal function should be adopted as exclusion criteria).
Choosing the best method to assess renal function should consider population characteristics such as age and BMI. Serum creatinine levels, anthropometric and clinical characteristics of patients are often used to estimate GFR. Body weight is an imperfect reflection of creatinine generation because increased body weight is associated more commonly with an increase in body fat or body water, edematous disorders, rather than an increase in muscle mass58,59. Creatinine clearance is not recommended when obese subjects are involved, but would be advised to exclude individuals that present creatinine level higher than 250 mmol/l14. A decline in renal function (creatinine clearance) occurs with advancing aging. Interestingly, L/M ratio did not change with aging due to a parallel progressive decline in the ability to excrete both lactulose and mannitol with increasing age60.
The use of the ratio L/M may not detect differences in IP between groups if one considers the possibility that an individual may be absorbing and excreting proportionally higher quantities of both mannitol and lactulose. Although this is only a hypothesis, obese women showed higher lactulose excretion, a tendency to higher mannitol excretion, while L/M ratio was not significantly different from lean women61. It is critical to assess the L/M ratio, as well as lactulose and mannitol recoveries separately, when interpreting test results62. Ferraris & Vinnakota63 showed in animal model that genetic obesity is associated with increased intestinal growth, which augments absorption of all types of nutrients. Obese men with chronic hyperglycemia showed evidence of increased small intestinal enterocyte mass (higher plasma citrulline) and increased enterocyte loss (higher plasma intestinal fatty acid binding proteins, I-FABP), but IP was not assessed64. Circulating levels of insulin which is a hormone usually increased in obese subjects65, may also influence IP. The addition of insulin in a cell culture showed that the insulin-induced decline in transcellular resistance is receptor-mediated and that receptors are localized in the basolateral membrane. Increased mannitol flux was an observed effect paralleled to this altered paracellular permeability66.
Barrier dysfunction may not be expressed all the time in particular conditions. It can range from mild to severe dysfunction (manifesting continuously) or intermittent dysfunction (manifesting only when the intestine is challenged). This susceptibility to barrier dysfunction can be detected using a 'challenge' test, as established by Hilsden and co-workers using aspirin67. Accordingly, subjects are given 1300 mg of aspirin (four 325 mg tablets) the night before the test and again on the morning of ingestion of the probe mixture. The use of the aspirin challenge showed that patients with non-alcoholic steatohepatitis do not have abnormal IP all the time, but they could easily develop gut leakiness when they are exposed to intestinal barrier stressors such as aspirin68.
Of note is the discussion presented recently by Vojdani69 in his review entitled "For assessment of intestinal permeability, size matters". Mannitol and lactulose are considered small molecules. Their use for IP assessment will not necessarily indicates structural damage in the TJ barrier, which would in turn allow penetration of large molecules. The use of probes of higher size (polysugars of 12,000- to -15,000 Da) may be more suitable to extrapolate if IP is higher enough to allow macromolecules such as bacterial toxins (such as lipopolysaccharides) and food antigens to permeate. Small inert markers may not mimic large molecules because of the size selectivity of TJ69.
Additional markers to indicate alteration in barrier function
There are other markers that could be associated to IP tests to improve the interpretation of dysfunctions of gut barrier. D-lactate is produced from carbohydrate fermentation by abnormal microbiota or when the number of bacteria elevates rapidly (bacterial overgrowth and short bowel syndrome)70-72. Plasma D-lactate had the lowest false-negative rate among C-reactive protein level and leukocyte counts to diagnose appendicitis, and acute inflammatory disorder73.
Circulating citrulline is an amino acid produced from glutamine by differentiated small intestinal enterocytes. Citrulline is a non-protein amino acid that seems to exert an important role in preserving gut barrier function and reducing bacterial translocation74. The circulating levels are dependent only on de novo synthesis from intestinal metabolic activity. It reflects the functional enterocyte mass and can be used as a biological tool to quantitatively investigate epithelial integrity and follow intestinal adaptation (i.e., post-surgical) at the enterocyte level. Loss of small bowel epithelial cell mass results in declined circulating levels of citrulline, such as for short bowel syndrome, chronic villous atrophy and chemotherapy75. Another situation in which the citrulline availability is decreased was shown to be during the course of induced endotoxemia in rats76. There some studies using animal models that show an association between endotoxemia and increased IP77-79. As citrulline is metabolized into arginine by kidney cells, the interpretation of its levels in patients with compromised renal function should not be reliable80.
The quantification of claudin-3 in the urine showed that its rapid appearance in this fluid correlated with immunohistochemically visualized loss of claudin-3, which is a major sealing TJ protein. Measurement of urinary claudin-3 can be used as noninvasive marker for intestinal TJ loss81.
The assessment of urinary concentration of endogenous cytosolic enterocyte proteins such as I-FABP and liver FABP (L-FABP) are potentially useful in reflecting enterocyte damage. Pelsers and co-workers investigated the distribution of these proteins in segments of human intestine82. They showed similar pattern of tissue distribution along the duodenal to colonal axis, being the jejunum the segment with highest content. In each intestinal segment it is observed a more than 40fold higher content of L-FABP than I-FABP. Elevated plasma levels of both proteins were found in patients with intestinal diseases82. Since FABP are small, water-soluble cytosolic proteins, the loss of enterocyte membrane integrity will lead to release of these proteins into the circulation71,83. FABP are expressed in cells on the upper part of the villi. Thus, destruction of these cells can lead to increased release of these proteins to the circulation. Results from a pilot study with celiac patients showed that circulating levels of FABP are significantly elevated in untreated patients with biopsy proven celiac disease compared with healthy controls84.
Local inflammation is associated with increased IP. An increased migration of granulocytes into the intestinal mucosa, usually due to conditions of inflammation, might result in the degranulation of their secondary granules, resulting in an increase in their proteins in feces85. Neutrophil derived proteins such as calprotectin, lactoferrin85-88 and elastase89 can be present in stool and also in plasma as a marker of inflammation90.
Finally, zonulin is a protein that exhibits the ability to reversibly modulate intercellular TJ similar to the toxin from Vibrio cholera known as zonula occluden toxin91,92. Proteomic analyses characterized zonulin as pre-haptoglobulin-2 (pre-HP2), a multifunctional protein that contains growth factor-like repeats. In its single-chain form, zonulin has the molecular conformation required to induce TJ disassembly by indirect transactivation via proteinase-activated receptor-292. Higher levels of zonulin are associated with disorders such as celiac disease and type 1 diabetes, and positive correlation between zonulin and IP has been demonstrated92,93.
Conclusion
There are many clinical situations in which increased IP seems to be present. If this alteration is contributing to worsen the clinical condition of affected subjects is still a question without answer for different diseases. This field of research should be better explored. However, the possible pitfalls should be taken into account. It is important to consider the different factors that may influence IP tests result and there are open questions regarding renal function and body size that should be further tested. This could help to produce more consistent evidences. The use of larger probes may be more appropriate to affirm that macromolecules such as food antigens and bacterial derived-compounds are crossing the barrier. Besides the use of IP tests, the association with the mentioned markers would be also interesting to investigate the role of barrier function in different diseases.
Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
1. Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis 2009; 27: 443-9. [ Links ]
2. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9: 799-809. [ Links ]
3. Menard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 2010; 3: 247-59. [ Links ]
4. Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol 2012; 46: S12-S7. [ Links ]
5. Buret AG. How stress induces intestinal hypersensitivity. Am J Pathol 2006; 168: 3-5. [ Links ]
6. Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012; 42: 71-8. [ Links ]
7. Tibbie JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002; 123: 450-60. [ Links ]
8. Bjarnason I, Macpherson A, Hollander D. Intestinal permeability: An overview. Gastroenterology 1995; 108: 1566-81. [ Links ]
9. Farhadi A, Banan ALI, Fields J, Keshavarzian ALI. Intestinal barrier: An interface between health and disease. J Gastroenterol Hepatol 2003; 18: 479-97. [ Links ]
10. Pirlich M, Norman K, Lochs H, Bauditz J. Role of intestinal function in cachexia. Curr Opin Clin Nutr Metab Care 2006; 9: 603-6. [ Links ]
11. Karaeren Z, Akbay A, Demirtas S, Ergüder I, Özden A. A reference interval study of urinary lactulose excretion: a useful test of intestinal permeability in adults. Turk J Gastroenterol 2002; 13: 35-9. [ Links ]
12. Paroni R, Fermo I, Molteni L, Folini L, Pastore MR, Mosca A, et al. Lactulose and mannitol intestinal permeability detected by capillary electrophoresis. J Chromatogr B 2006; 834: 183-7. [ Links ]
13. Lostia AM, Lionetto L, Principessa L, Evangelisti M, Gamba A, Villa MP, et al. A liquid chromatography/mass spectrometry method for the evaluation of intestinal permeability. Clinical Biochemistry 2008; 41: 887-92. [ Links ]
14. Duerksen DR, Wilhelm-Boyles C, Parry DM. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci 2005; 50: 785-90. [ Links ]
15. Uil JJ, van Elburg RM, van Overbeek FM, Mulder CJ, Van Berge-Henegouwen GP, Heymans HS. Clinical implications of the sugar absorption test: intestinal permeability test to assess mucosal barrier function. Scand J Gastroenetrol Suppl 1997; 223: 70-8. [ Links ]
16. Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006; 55: 1512-20. [ Links ]
17. Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 2011; 141: 769-76. [ Links ]
18. Matter K, Balda MS. Signalling to and from tight junctions. Nature Rev 2003; 4: 225-36. [ Links ]
19. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470-81. [ Links ]
20. Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877-87. [ Links ]
21. Vilela E, Torres HOG, Ferrari MLA, Lima AS, Cunha A. Gut permeability to lactulose and mannitol differs in treated Crohn's disease and celiac disease patients and healthy subjects. Braz J Med Biol Res 2008; 41: 1105-9. [ Links ]
22. Teshima C, Meddings J. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep 2008; 10: 443-9. [ Links ]
23. Watson AJM, Duckworth CA, Guan Y, Montrose MH. Mechanisms of epithelial cell shedding in the mammalian intestine and maintenance of barrier function. Ann N Y Acad Sci 2009; 1165: 135-42. [ Links ]
24. Camilleri M, Nadeau A, Lamsam J, Linker Nord S, Ryks M, Burton D, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 2010; 22: e15-e26. [ Links ]
25. Uil JJ, VanElburg RM, VanOverbeek FM, Mulder CJJ, Vanberge Henegouwen GP, Heymans HSA. Clinical implications of the sugar absorption test: Intestinal permeability test to assess mucosal barrier function. Scand J Gastroenterol 1997; 32: 70-8. [ Links ]
26. Meddings JB, Sutherland LR, Byles NI, Wallace JL. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology 1993;104: 1619-26. [ Links ]
27. Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 1998; 114: 83-92. [ Links ]
28. Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A. Gas chromatographic method for detection of urinary sucralose: application to the assessment of intestinal permeability. J Chromatogr B 2003; 784: 145-54. [ Links ]
29. Anderson ADG, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand 2004; 182: 171-7. [ Links ]
30. McOmber ME, Ou C-N, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr 2010; 50: 269-75. [ Links ]
31. Akram S, Mourani S, Ou C-N, Rognerud C, Sadiq R, Goodgame R. Assessment of intestinal permeability with a two-hour urine collection. Dig Dis Sci 1998; 43: 1946-50. [ Links ]
32. Cox MA, Iqbal TH, Cooper BT, Lewis KO. An analytical method for the quantitation of mannitol and disaccharides in serum: a potentially useful technique in measuring small intestinal permeability in vivo. Clin Chim Acta 1997; 263: 197-205. [ Links ]
33. Fleming SC, Duncan A, Russell RI, Laker MF. Measurement of sugar probes in serum: an alternative to urine measurement in intestinal permeability testing. Clin Chem 1996; 42: 445-8. [ Links ]
34. Martinez-Augustin O, Boza JJ, Romera JM, Gil A. A rapid gasliquid chromatography method for the determination of lactulose and mannitol in urine: Clinical application in studies of intestinal permeability. Clin Biochem 1995; 28: 401-5. [ Links ]
35. Van Der Hulst RRWJ, Von Meyenfeldt MF, Van Kreel BK, Thunnissen FBJM, Brummer R-JM, Arends J-W, et al. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 1998; 14: 1-6. [ Links ]
36. Dastych M, Dastych M Jr, Novotná H, Číhalová J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn's disease. Dig Dis Sci 2008; 53: 2789-92. [ Links ]
37. Marsilio R, D'Antiga L, Zancan L, Dussini N, Zacchello F. Simultaneous HPLC determination with light-scattering detection of lactulose and mannitol in studies of intestinal permeability in pediatrics. Clin Chem 1998; 44: 1685-91. [ Links ]
38. Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol 2009; 44: 23-9. [ Links ]
39. Smecuol E, Pinto Sanchez MI, Suarez A, Argonz JE, Sugai E, Vazquez H, et al. Low-dose aspirin affects the small bowel mucosa: results of a pilot study with a multidimensional assessment. Clin Gastroenterol Hepatol 2009; 7: 524-9. [ Links ]
40. Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol 2008; 42: 349-61. [ Links ]
41. Kavanaugh MJ, Clark C, Goto M, Kovacs EJ, Gamelli RL, Sayeed MM, et al. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns 2005; 31: 290-6. [ Links ]
42. Pals KL, Chang R-T, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol 1997; 82: 571-6. [ Links ]
43. Söderholm JD, Perdue MH. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 2001; 280: G7-G13. [ Links ]
44. Saunders P, Santos J, Hanssen NM, Yates D, Groot J, Perdue M. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci 2002; 47: 208-15. [ Links ]
45. Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology 1997; 113: 802-7. [ Links ]
46. May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology 1993; 104: 1627-32. [ Links ]
47. Hollander D. Permeability in Crohn s disease: altered barrier functions in healthy relatives? Gastroenterology 1993; 104: 1848-51. [ Links ]
48. Travis S, Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci 1992; 82: 47l-88. [ Links ]
49. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 2003; 52: 439-51. [ Links ]
50. Celli M, D'Eufemia P, Dommarco R, Finocchiaro R, Aprigliano D, Martino F, et al. Rapid gaschromatographic assay of lactulose and mannitol for estimating intestinal permeability. Clin Chem 1995; 41: 752-6. [ Links ]
51. Farhadi A, Keshavarzian A, Fields JZ, Sheikh M, Banan A. Resolution of common dietary sugars from probe sugars for test of intestinal permeability using capillary column gas chromatography. J Chromatogr B 2006; 836: 63-8. [ Links ]
52. Mattioli F, Fucile C, Marini V, Isola L, Montanaro F, Savarino V, et al. Assessment of intestinal permeability using sugar probes: influence of urinary volume. Clin Lab 2011; 57: 909-18. [ Links ]
53. Lin HC, Elashoff JD, Kwok GM, Gu YG, Meyer JH. Stimulation of duodenal motility by hyperosmolar mannitol depends on local osmoreceptor control. Am J Physiol Gastrointest Liver Physiol 1994; 266: G940-G3. [ Links ]
54. Barboza Jr MS, Silva TMJ, Guerrant RL, Lima AAM. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz J Med Biol Res 1999; 32: 1499-504. [ Links ]
55. Maduka IC, Neboh EE, Shu EN, Ikekpeazu EJ. Drug dosing in adult and paediatric population in developing countries: possible pharmaceutical misadventure. Br J Pharm Toxicol 2010; 1: 77-80. [ Links ]
56. Bosma RJ, Homan JJ, Heide vd, Oosterop EJ, Jong PEd, Navis G. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int 2004; 65: 259-65. [ Links ]
57. Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The Effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 2003; 14: 1480-6. [ Links ]
58. Agarwal R. Estimating GFR from serum creatinine concentration: Pitfalls of GFR-estimating equations. Am J Kidney Dis 2005;45:610-3. [ Links ]
59. Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis 2005; 46: 233-41. [ Links ]
60. Saltzman JR, Kowdley KV, Perrone G, Russell RM. Changes in small intestine permeability with aging. J Am Geriatr Soc 1995;43:160-4. [ Links ]
61. Teixeira TFS, Souza NCS, Chiarello PG, Franceschini SCC, Bressan J, Ferreira CLLF, et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr 2012; 31: 735-40. [ Links ]
62. Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 2013. [ Links ]
63. Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr 1995; 62: 540-6. [ Links ]
64. Verdam FJ, Greve JWM, Roosta S, van Eijk H, Bouvy N, Buurman WA, et al. Small intestinal alterations in severely obese hyperglycemic subjects. JCEM 2011; 96: E379-E83. [ Links ]
65. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840-6. [ Links ]
66. McRoberts JA, Aranda R, Riley N, Kang H. Insulin regulates the paracelular permeability of cultured intestinal epithelial cell monolayers. J Clin Invest 1990; 85: 1127-34. [ Links ]
67. Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology 1996; 110: 1395-403. [ Links ]
68. Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int 2008; 28: 1026-33. [ Links ]
69. Vojdani A. For the assessment of intestinal permeability, size matters. Altern Ther Health Med 2013; 19: 12-24. [ Links ]
70. Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol 2006; 4: 11-20. [ Links ]
71. Derikx JPM, Luyer MDP, Heineman E, Buurman WA. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol 2010; 16: 5272-9. [ Links ]
72. Talasniemi JP, Pennanen S, Savolainen H, Niskanen L, Liesivuori J. Analytical investigation: Assay of d-lactate in diabetic plasma and urine. Clin Biochem 2008; 41: 1099-103. [ Links ]
73. Çağlayan F, Çakmak M, Çağlayan O, Çavuşogˇlu T. Plasma dlactate levels in diagnosis of appendicitis. J Invest Surg 2003; 16: 233-7. [ Links ]
74. Batista MA, Nicoli JR, dos Santos Martins F, Nogueira Machado JA, Esteves Arantes RM, Pacifico Quirino IE, et al. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. JPEN 2012; 36: 69-76. [ Links ]
75. Blijlevens NMA, Lutgens LCHW, Schattenberg AVMB, Donnelly JP. Citrulline: a potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant 2004; 34: 193-6. [ Links ]
76. Elwafi F, Curis E, Zerrouk N, Neveux N, Chaumeil J-C, Arnaud P, et al. Endotoxemia affects citrulline, arginine and glutamine bioavailability. Eur J Clin Invest 2012; 42: 282-9. [ Links ]
77. Brun P, Castagliuolo I, Leo VD, Buda A, Pinzani M, Palu G, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2007; 292: G518-G25. [ Links ]
78. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007; 50: 2374-83. [ Links ]
79. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009; 58: 1091-103. [ Links ]
80. Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008;27:328-39. [ Links ]
81. Thuijls G, Derikx JPM, Haan J-Jd, Grootjans J, Bruïne Ad, Masclee AAM, et al. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol 2010; 44: e14-e9. [ Links ]
82. Pelsers MMAL, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003; 36: 529-35. [ Links ]
83. Grootjans J, Thuijls G, Verdam F, Derikx JPM, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastroenterol 2010; 2: 61-9. [ Links ]
84. Derikx JPM, Vreugdenhil ACE, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JGMC, et al. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol 2009; 43: 727-33. [ Links ]
85. Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15: 1746-54. [ Links ]
86. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Diss 2006; 12: 524-34. [ Links ]
87. Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, Boone JH, et al. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2007; 44: 414-22. [ Links ]
88. Joishy M, Davies I, Ahmed M, Wassel J, Davies K, Sayers A, et al. Fecal Calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2009; 48: 48-54. [ Links ]
89. Mania-Pramanik J, Potdar SS, Vadigoppula A, Sawant S. Elastase: A predictive marker of inflammation and/or infection. J Clin Lab Anal 2004; 18: 153-8. [ Links ]
90. Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, et al. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis 2005; 11: 1085-91. [ Links ]
91. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011; 91: 151-75. [ Links ]
92. Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. PNAS 2009; 106: 16799-804. [ Links ]
93. Duerksen DR, Wilhelm-Boyles C, Veitch R, Kryszak D, Parry DM. A Comparison of antibody testing, permeability testing, and zonulin levels with small-bowel biopsy in celiac disease patients on a gluten-free diet. Dig Dis Sci 2010; 55: 1026-31. [ Links ]
94. Dupont C, Barau E, Molkhou P, Raynaud F, Barbet JP, Dehennin L. Food-induced alterations of intestinal permeability in children with cow's milk-sensitive enteropathy and atopic dermatitis. J Pediatr Gastroenterol Nutr 1989; 8: 459-65. [ Links ]
95. Hossain MI, Nahar B, Hamadani JD, Ahmed T, Roy AK, Brown KH. Intestinal mucosal permeability of severely underweight and non-malnourished Bangladeshi children, and effects of nutritional rehabilitation. J Pediatr Gastroenterol Nutr 2010; 51: 638-44. [ Links ]
96. Raj SM, Sein KT, Anuar AK, Mustaffa BE. Effect of intestinal helminthiasis on intestinal permeability of early primary schoolchildren. Trans Rl Soc Trop MedHyg 1996; 90: 666-9. [ Links ]
97. Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou C-N. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 2008; 153: 646-50. [ Links ]
98. Ventura MT, Polimeno L, Amoruso AC, Gatti F, Annoscia E, Marinaro M, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis 2006; 38: 732-6. [ Links ]
99. Nagpal K, Minocha VR, Agrawal V, Kapur S. Evaluation of intestinal mucosal permeability function in patients with acute pancreatitis. Am J Surg 2006; 192: 24-8. [ Links ]
100. de la Maza MP, Gotteland M, Ramirez C, Araya M, Yudin T, Bunout D, et al. Acute nutritional and intestinal changes after pelvic radiation. J Am Coll Nutr 2001; 20: 637-42. [ Links ]
101. Secondulfo M, Iafusco D, Carratù R, deMagistris L, Sapone A, Generoso M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis 2004; 36: 35-45. [ Links ]
102. Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006; 55: 1443-9. [ Links ]
103. Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007; 50: 1561-9. [ Links ]
104. Goebel A, Buhner S, Schedel R, Lochs H, Sprotte G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology 2008;47: 1223-7. [ Links ]
105. Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 1999; 94: 200-7. [ Links ]
106. Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, et al. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis 2010; 42: 200-4. [ Links ]
107. Liu H, Zhang S, Yu A, Qu L, Zhao Y, Huang H, et al. Studies on intestinal permeability of cirrhotic patients by analysis lactulose and mannitol in urine with HPLC/RID/MS. Bioorg Med Chem Lett 2004; 14: 2339-44. [ Links ]
108. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009;146: 41-6. [ Links ]
109. Smecuol E, Sugai E, Niveloni S, Vázquez H, Pedreira S, Mazure R, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol 2005; 3: 335-41. [ Links ]
110. Vilela E, Abreu Ferrari M, Gama Torres H, Martins F, Goulart E, Lima A, et al. Intestinal permeability and antigliadin antibody test for monitoring adult patients with celiac disease. Dig Dis Sci 2007;52: 1304-9. [ Links ]
111. Fries W, Renda MC, Lo Presti MA, Raso A, Orlando A, Oliva L, et al. Intestinal permeability and genetic determinants in patients, first-degree relatives, and controls in a high-incidence area of Crohn's disease in Southern Italy. Am J Gastroenterol 2005; 100: 2730-6. [ Links ]
112. Benjamin J, Makharia GK, Ahuja V, Kalaivani M, Joshi YK. Intestinal permeability and its association with the patient and disease characteristics in Crohn s disease. World J Gastroenterol 2008; 14: 1399-405. [ Links ]
113. Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor B-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol 2009; 587: 3317-28. [ Links ]
114. Homma H, Hoy E, Xu D-Z, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol 2005; 288: G466-G72. [ Links ]
115. Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, et al. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol 2008; 126: 67-80. [ Links ]
116. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and Tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 2005; 166: 409-19. [ Links ]
117. Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA Regulation of intestinal epithelial tight junction permeability. Gastroenterology 2011;141:1323-33. [ Links ]
118. Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med 2008; 205: 897-913. [ Links ]
119. Parodi A, Lauritano EC, Nardone G, Fontana L, Savarino V, Gasbarrini A. Small intestinal bacterial overgrowth. Dig Liver Dis Suppl 2009; 3: 44-9. [ Links ]
120. Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 2012; 303: G32-G41. [ Links ]
121. Zareie M, Johnson-Henry K, Jury J, Yang P-C, Ngan B-Y, McKay DM, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006; 55: 1553-60. [ Links ]
122. Liu Z, Zhang P, Ma Y, Chen H, Zhou Y, Zhang M, et al. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the intestinal permeability. Mol Bio Rep 2011; 38: 1353-61. [ Links ]
123. Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003; 52: 988-97. [ Links ]
124. Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 2006; 130: 731-46. [ Links ]
125. Fedwick JP, Lapointe TK, Meddings JB, Sherman PM, Buret AG. Helicobacter pylori activates myosin ligt-chain kinase to disrupt claudin-4 and claudin-5 and increase epithelial permeability. Infect Immun 2005; 73: 7844-52. [ Links ]
126. Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. Cellular Retinoic Acid Bioavailability Determines Epithelial Integrity: Role of Retinoic Acid Receptor alpha Agonists in Colitis. Mol Pharmacol 2007; 71: 250-8. [ Links ]
127. Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Incà R. Zinc supplementation tightens "Leaky Gut" in Crohn's disease. Inflamm Bowel Dis 2001; 7: 94-8. [ Links ]
128. Chen P, Soares AM, Lima AAM, Gamble MV, Schorling JB, Conway M, et al. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. JHPN 2003; 21: 309-15. [ Links ]
129. Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4alpha mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol 2010; 299: G643-G51. [ Links ]
130. Willemsen LM, Koetsier M, Balvers M, Beermann C, Stahl B, Tol EF. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur J Nutr 2008; 47: 183-91. [ Links ]
131. Li Q, Zhang Q, Wang M, Zhao S, Xu G, Li J. n-3 polyunsaturated fatty acids prevent disruption of epithelial barrier function induced by proinflammatory cytokines. Mol Immunol 2008; 45: 1356-65. [ Links ]
132. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008; 294: G208-G16. [ Links ]
133. Pachikian BD, Neyrinck AM, Deldicque L, De Backer FC, Catry E, Dewulf EM, et al. Changes in Intestinal bifidobacteria levels are associated with the inflammatory response in magnesium-deficient mice. J Nutr 2010; 140: 509-14. [ Links ]
134. Mazzon E, Sturniolo GC, Puzzolo D, Frisina N, Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut 2002; 51: 507-13. [ Links ]
135. Boudry G, Jury J, Yang PC, Perdue MH. Chronic psychological stress alters epithelial cell turn-over in rat ileum. Am J Physiol Gastrointest Liver Physiol 2007; 292: G1228-G32. [ Links ]
136. Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab 2010; 7: 19. [ Links ]
137. Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol 2012; 18: 923-9. [ Links ]
138. Gao Y, He L, Katsumi H, Sakane T, Fujita T, Yamamoto A. Improvement of intestinal absorption of water-soluble macromolecules by various polyamines: Intestinal mucosal toxicity and absorption-enhancing mechanism of spermine. Int J Pharm 2008; 354: 126-34. [ Links ]
139. Frias R, Strube K, Ternes W, Collado MC, Spillmann T, Sankari S, et al. Comparison of 51Chromium-labeled ethylene-diamine tetra-acetic acid and iohexol as blood markers for intestinal permeability testing in Beagle dogs. Vet J 2012; 192: 123-5. [ Links ]
140. Andersen R, Stordahl A, Aase S, Laerum F. Intestinal permeability of X-ray contrast media iodixanol and iohexol during bacterial overgrowth of small intestines in rats. Dig Dis Sci 2001;46:208-13. [ Links ]
141. Paseephol T, Small DM, Sherkat F. Lactulose production from milk concentration permeate using calcium carbonate-based catalysts. Food Chem 2008; 111: 283-90. [ Links ]
142. Olano A, Corzo N. Lactulose as a food ingredient. J Sci Food Agric 2009; 89: 1987-90. [ Links ]
143. Rupérez P, Toledano G. Celery by-products as a source of mannitol. Eur Food Res Technol 2003; 216: 224-6. [ Links ]
144. Stoop JMH, Williamson JD, Mason Pharr D. Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci 1996;1:139-44. [ Links ]
145. Mäkinen KK, Söderllng EVA. A quantitative study of mannitol, sorbitol, xylitol, and xylose in wild berries and commercial fruits. J Food Sci 1980; 45: 367-71. [ Links ]
146. Binns NM. Sucralose - all sweetness and light. Nutr Bull 2003; 28: 53-8. [ Links ]
![]() Correspondence:
Correspondence:
Tatiana Fiche Salles Teixeira.
Departamento de Nutrição e Saúde.
Universidade Federal de Viçosa.
Av. Ph. Rolfs. Campus Universitário.
36570-000 Viçosa. Minas Gerais. Brasil.
E-mail: tatifichee@hotmail.com
Recibido: 19-VIII-2013.
1.a Revisión: 25-X-2013.
Aceptado: 15-XI-2013.