My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.33 n.5 Madrid Sep./Oct. 2016
https://dx.doi.org/10.20960/nh.584
Lifestyle and vitamin D dosage in women with breast cancer
Relación de la vitamina D y el ejercicio físico en mujeres con cáncer de mama
Catarina Maria Nogueira Oliveira-Sediyama1,4, Manoela Maciel dos Santos Dias1, Milene Cristine Pessoa1, Andréia Ribeiro Queiroz1, Lara Gomes Suhett1, Renata Nascimento Freitas2, Sérgio Oliveira de Paula3 and Maria do Carmo Gouveia Peluzio1
1Department of Nutrition and Health. Laboratory of Nutrition Biochemistry. Federal University of Viçosa. University Campus, Viçosa. Brazil.
2Center of Biological Sciences Reseach-NUPEB. Molecular Epidemiology Laboratory. Federal University of Ouro Preto. Ouro Preto, Brazil.
3Department of Animal Biology. Federal University of Viçosa. University Campus. Viçosa, Brazil.
4Department of Nursing and Medicine. Federal University of Viçosa. University Campus. Viçosa, Brazil
The authors would like to thank the National Counsel of Technological and Scientific Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Minas Gerais State Research Foundation (FAPEMIG) from Brazil for financially supporting this project.
ABSTRACT
Introduction: The prevention strategy of breast cancer is still the key factor for early diagnosis and the most effective method for tracking the disease.
Objective: This study aimed to evaluate the association vitamin D level with breast cancer in women.
Methods: This hospital case-control study was conducted with 181 women with breast cancer and 197 healthy controls. Vitamin D status, calcium, phosphorus and PTH serum dosage and data collection related to lifestyle and patient's history, besides anthropometric measurements were performed. Univariate analysis (Chi-square and raw odds ratio) and multivariate analysis were performed through multivariate logistic regression.
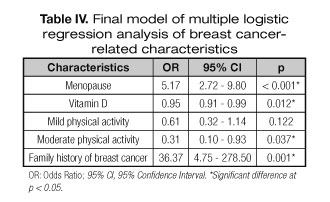
Results: This study shows a higher value of vitamin D in health controls (26.9 mg/dL) than in breast cancer women (24.8 mg/dL). Higher numbers of women with sufficient vitamin D status (34.85%) were found in control group than cancer group. Using the multiple logistic regression model, the family history of breast cancer (OR 36.37, 95%CI 4.75-278.50) and menopause (OR 5.17, 95% CI 2.72-9.80) had a direct association with breast cancer, while the level of vitamin D (OR 0.95, 95%CI 0.91-0.99) and moderate physical activity (OR 0.31, 95%CI 0.10-0.93) maintained the inverse associations with the disease.
Conclusion: Vitamin D status and the practice of moderate physical activity were considered protective factors for breast cancer. However, menopause and family history of breast cancer were considered a risk factor for breast cancer.
Key words: Breast cancer. Vitamin D. Menopause. Physical activity. Case-control study.
RESUMEN
Introducción: la estrategia de prevención del cáncer de mama sigue siendo el factor clave para el diagnóstico precoz y el método más eficaz para el seguimiento de la enfermedad.
Objetivo: este estudio tuvo como objetivo evaluar el nivel de vitamina D asociado con el cáncer de mama en las mujeres.
Métodos: este estudio de casos y controles hospitalarios se llevó a cabo con 181 mujeres con cáncer de mama y 197 controles sanas. Se estudió el nivel de vitamina D, calcio, fósforo y la dosis de suero PTH; se recopilaron de datos relacionados con el estilo de vida y com la historia de las pacientes, además se realizaron mediciones antropométricas. El análisis univariante (Chi-cuadrado probabilidades y primas ratio) y el análisis multivariado se realizó mediante regresión logística multivariante.
Resultados: este estudio muestra un valor más alto de vitamina D en los controles de salud (26,9 mg/dl) que en las mujeres con cáncer de mama (24,8 mg/dl). Se encontraron más mujeres con suficiente vitamina D (34,85%) en el grupo control que en el grupo de cáncer. Usando el modelo de regresión logística múltiple, la historia familiar de cáncer de mama (OR 36,37; IC del 95%: 4,75 a 278,50) y la menopausia (OR 5,17; IC del 95%: 2,72 a 9,80) se halló una relación directa con el cáncer de mama, mientras que el nivel de vitamina D (OR 0,95; IC del 95%: 0,91 a 0,99) y la actividad física moderada (OR 0,31; IC del 95%: 0,10 a 0,93) mantienen las asociaciones inversas con la enfermedad.
Conclusión: el estado de vitamina D y la práctica de actividad física moderada se consideraron factores de protección para el cáncer de mama. Sin embargo, la menopausia y la historia familiar de cáncer de mama se consideran un factor de riesgo para el cáncer de mama.
Palabras clave: Cáncer de mama. Vitamina D. Menopausia. Actividad física. Estudio de casos y controles.
Introduction
Increasing life expectancy, adoption of western lifestyle, and growing urbanization has raised the global incidence of breast cancer (1). Breast cancer has known risk factors such as the cellular aging, family history, alcohol consumption (2), overweight (3), sedentary lifestyle, and high breast tissue density (4). Other risk factors are related to women's reproductive life, which leads to increased endogenous estrogen levels, as the later age at first childbirth, early menarche, and late menopause (1,3). Some studies indicated that sun exposure and vitamin D levels are inversely proportional to the risk of developing breast cancer (5,6). Moreover, it is suggested a greater benefit if sun exposure occurs during the breast tissue development in young stages of women's life (7). Furthermore, vitamin D may act directly in tumorigenesis, and extrarenal tissues such as breast tissue, expressing the CYP27B1 enzyme, that provide instructions for the synthesis of 1-α-hydroxylase. This enzyme is responsible for converting the inactive precursor of vitamin D to the active form, namely, 1, 25-dihydroxycholecalciferol [1,25(OH)2D3]. This vitamin can have autocrine or paracrine activity, protecting the breast tissue cells from malignant transformation (8). The production of 1,25(OH)2D3 is controlled by its own levels and is controlled by the parathyroid hormone, fetal growth factor 23, and calcium and phosphorus levels in serum (9).
Parathyroid hormone (PTH) along with vitamin D is involved in calcium homeostasis acting directly or indirectly in organs related to its storage, excretion, and absorption. Moreover, PTH can promote both formation and reabsorption of the bone tissue. Also, drops in calcium levels of 10% are sufficient to increase PTH serum levels (10,11).
In this study, we aimed to evaluate the associations between lifestyle, vitamin D status, PTH, calcium and phosphorus levels in serum, with breast cancer in women in the Hospital Foundation of Belo Horizonte, Minas Gerais, Brazil.
Material and methods
STUDY POPULATION
This study is a part of hospital-based case-control study, whose source of patients were health services, ambulatory care; hospital; compulsory notification or registration diseases. The study was conducted with women treated in the Mastology Service of Odete Valadares Maternity, of Hospital Foundation of Minas Gerais State (FHEMIG), in Belo Horizonte, Minas Gerais, Brazil. Women who were referred to the ambulatory care were invited to participate in the study as volunteers. Those women who accepted signed the free and clarified consent term. The study followed the Declaration of Helsinki and was approved by the National Committee of Ethics in research (protocol number: 1889/2005) (12,13).
The case group (GCa) was composed by women who were referred for evaluation at the Maternity Odete Valadares, older than 18 years, living in rural or metropolitan region of Belo Horizonte, Minas Gerais, Brazil, with mammography result BI-RADS (14) (Breast Imaging Reporting and Data System) 0, 3, 4 or 5 (assessment incomplete, probably benign, suspicious abnormality and highly suspicious of malignancy, respectively) and breast cancer confirmed by biopsy. The control group (GCo) was composed by women who were older than 18 years old, lived in same area in Brazil, and mammography's result BI-RADS 1 or 2 (negative and benign findings) (14).
The exclusion criteria were: the age below 18 years old, previous history of any other cancer type, benign, suspicious or indeterminate biopsy result or not having performed mammography.
A total of 378 women were selected for the study, 181 healthy women (GCo group) and 197 women with breast cancer (GCa group). Only those women with ductal or invasive lobular carcinoma were included in GCa group. Women with in situ disease, or Phyllodes malignant or borderline breast tumor or benign disease biopsy were not included in the study.
STUDY DESING
Data collection was carried out in the ambulatory in two steps: In the first step, prior to diagnosis, nutritional interview, lifestyle characteristics, medical history (such as diabetes), family history data and anthropometric measurements were collected. In the second stage, after diagnosis, peripheral blood sample was collected for laboratory analysis.
The anthropometric assessment was performed considering the weight and percentage of fat using the Tanita Body Fat Monitor Scale (model TBF 531©) and height was noted using the vertical stadiometer (Altura Exata©). The waist and hip circumferences were also measured and the body mass index (BMI) was calculated (12,15).
A questionnaire previously validated by Pena et al (12) was applied to the studied population to characterize the sample with regards to socioeconomic issues, medical history (such as diabetes), lifestyle, and food consumption.
Regarding the variables related to women's gynecological history, menarche and menopause age were requested. If they had children, the number of living children, abortion, breastfeeding, duration of breastfeeding for each child, age at first pregnancy, oral contraceptive use, and hormone replacement therapy were also asked.
The patients provided information about lifestyle, as alcohol use, smoking behavior and physical activity Those women who consumed at least one dose (10 g of alcohol) of any alcoholic beverage daily or more than 3 days a week were considered alcoholics (12,16). Those who reported smoking at least one cigarette per day, regardless the time of use, were considered smokers (17). The practice of physical activity was assessed by the International Physical Activity Questionnaire (IPAQ) (18), short version, and the women were classified as sedentary, low active, or active (12) (Fig. 1).
BIOCHEMICAL ANALYSES
Blood samples (4 mL) were collected in opaque bottles containing EDTA at the Odette Valadares Maternity's ambulatory care on interview day. The blood was centrifuged to obtain plasma and stored at -80 oC for further analysis.
Serum levels of vitamin D, calcium, phosphorus, and PTH were measured in 256 women. For the vitamin D dosage, the ABBOTT© chemiluminescence kit was used and the metabolite 25-hydroxyvitamin D [25(OH)D] was dosed (imprecision ≤ 10%,accuracy, 8,0 ng/mL detection limit, 8,0-160 ng/mL reference range). PTH was also dosed by chemiluminescence using the Beckman Couter© Kit (imprecision ≤ 8%, accuracy, 1 pg/mL detection limit, 12-88 pg/mL reference range). Calcium and phosphorus were measured by colorimetric method according to the manufacturer's protocol (Beckman Couter©) (calcium; precision 0,95% coefficient of variation, detection limit 0,01 mmol/L, reference range 8,8-10,6 mg/dL, phosphorus: precision < 3% CV, detection limit 1 mg/dL, reference range 3.7-7.2 mg/dL). The analyses were performed in the clinical laboratory of the Health Division at the Federal University of Viçosa, Minas Gerais, Brazil.
STATISTICAL ANALYSIS
The statistical analyses were carried out using Stata program, version 9.1. Kolmogorov Smirnov normality test was performed for each continous variable. The mean values for the variables net income, age, age at first pregnancy, age of menopause, patients' age when they performed the first mammography, weight, BMI, parathyroid hormone, calcium, phosphorus and vitamin D presented asymmetric distribution. Furthermore, Student's t test was used to evaluate the differences between the averages of the GCa and GCo groups. The Mann-Whitney test was used for determination of asymmetric distribution. In addition, the association between the breast cancer and each categorical variable was evaluated using the Chi-square test. Univariate analysis (chi-square and raw odds ratio) and multivariate analysis were performed through multivariate logistic regression (cancer was considered dependent variable, and menopause, diabetes, age at first pregnancy, patients age when they made the first mammography, physical activity, income, contraceptive, body fat, -vitamin D, alcoholism, breast cancer history family, nulliparity, age and breast-feeding were considered independent variables). A 5% level of significance was considered for the analysis.
Results
The characteristics of the women in each group are shown in table I. The women with breast cancer were older than healthy controls (p < 0.001). There was no difference between the groups regarding the level of education and rural or urban living. A total of 378 women were evaluated, with a median age of 51 years (44-59 years). Among them, 43.09% of women were from the rural zone and 29.63% had attended full middle school (full middle school, 7th grade, or more) (data not shown).
The average net income of the family and the minimum wage was assessed. Women from control group had higher income than women from case group (p = 0.018) (Table I). In relation to reproductive factors, more women from GCo reported having at least one born child alive than the women from GCa (p = 0.014) (Table I). There was no difference between the groups regarding breastfeeding, (p = 0.120) and use of hormone replacement therapy (p = 0.45). The contraceptive use was higher in control women (p = 0.025). The first term pregnancy equal to or more than 30 years old was higher in GCa group (p = 0.024) (Table I). The average of age of women when they first underwent mammography was higher in the GCa group (43 years × 40 years, p = 0.002). Approximately 80.51% of cases and 41.24% of controls were in menopause (p < 0.001) (Table I). However, no difference was observed for the age of menopause between the groups (p = 0.61). The mean age of menarche was the same in both groups approximately 13 years (p = 0.611) (data not shown).
Differences between groups were not found for anthropometric characteristics, like BMI, weight and waist-hip ratio (WHR) (p = 0.842, p = 0.402 and p = 0.464). To assess the percentage of body fat, patients were classified as normal/eutrophic body fat or high body fat. More women of control group presented high percentage of body fat than in GCa (p = 0.049) (Table I).
The lifestyle, current or previous smoking, alcoholism, and physical activity was assessed. In GCo group, 17.68% of women reported current smoking, while in GCa group, only 12.69% reported smoking (p = 0.014). With regard to alcoholism, more women in GCo group did not consume alcohol in sufficient levels to configure alcoholism than in GCa (p < 0.001) In relation to the practice of physical activity, most of women from control group practiced mild physical activity. However, in case group, most of the women did not practice any physical activity. In GCo 13.41% had moderate physical activity, while in the GCa group 3.57% reported the same (p = 0.001) (Table I).
The median value of vitamin D in GCo group was higher than GCa group (p = 0.008). In GCo group, 34.59% had vitamin D levels above 30 ng/mL, while in the GCa group, only 21.95% had sufficient levels. Approximately 51.13 and 51.22% of controls and cases, respectively, had vitamin D levels considered as insufficient (between 21 and 29.9 ng/mL); while 14.29% in GCo group and 26.83% in GCa group had deficient levels, less than 20 ng/mL (p = 0.014). The analysis of calcium, phosphorous, and PTH did not show any differences (p > 0.05) between the groups (Table II).
The results of the univariate analysis indicate a direct association between the disease and the age of women, nulliparity, age of first successful pregnancy, age the first mammography, diabetes mellitus, and family history of breast cancer. Inverse association was verified with moderate physical activity, percentage of body fat, use of hormonal contraceptive, and vitamin D levels (p < 0.05) (Table III).
Finally, a direct association was found between menopause (OR = 5.17, 95% CI = 2.72-9.80) and family history of breast cancer (OR = 36.37, 95% CI = 4.75-278.50), considered risk factors. Moreover, an inverse association was also observed between vitamin D levels (OR = 0.95, 95%CI = 0.91-0.99) and moderate physical activity (OR = 0.31, 95%CI = 0.10-0.93), with protective effect for breast cancer (Table IV).
Discussion
The results suggest that menopause (12 months or more of amenorrhea) is associated with breast cancer. GCa had more menopausal women than GCo. This result was maintained in the final statistical model, even after the adjusted model for patient age. Despite the breast cancer patients were older than controls, it did not influence the association between breast cancer and menopausal status.
Although menopause is not considered a risk factor for breast cancer, the late menopause (after 55 years) is directed associated with the disease (19), due to longer exposure of endogenous hormones. The estrogen exposure has also been associated with conditions like nulliparity, late menopause age, early menarche and giving first birth at later age (20). There is an increased risk each year of late menopause, particularly in positive estrogen receptors tumors (2121). Despite of it, this study did not show difference for the age of menopause (p = 0.600) and late menopause (p = 0.083) between GCa e GCo. This result is due the close menopause age between groups.
Age is considered a risk factor for breast cancer (22). In approximately 80% of cases, the disease occurs after age 50 (4). According to Howlader et al, the risk of developing breast cancer among American women increases 0.44% at 30 years, 2.38% at age 50 and 3.82% at 70 years (23). In this study, the patients of CaG had median age higher than controls, with a direct association with breast cancer. However, this association did not persist after the adjustment in the final model. In a systematic review, Cutler et al. (24) observed an average of cumulative breast cancer incidence of 0.22% each year of age in women less of 50 years and 0.23% per year among women either 50 years old or with surgically menopausal.
Family history of breast cancer is directly associated with the disease. Having a first degree relative with breast cancer is one of the most consistent risk factors for the disease (19,25). The breast cancer risk in lifetime is 7.8% in women without history family, and rises to 13.3 % with one case in family and 21.1% with two affected relatives (25). The increased risk of breast cancer is associated with different subtypes of the disease, both in cancers with hormone receptor positive and negative (26). Heredity accounts for about 27% of cases of breast cancer, for the sporadic cancer, most cases are related to the environment (27). Family history of breast cancer is also found in cases of hereditary breast cancer associated with gene mutations BRCA1 and BRCA2, and other mutations, accounting for 5-10% of cases (28).
In our study more women in GCa were reported with family history of breast cancer than in GCo (p < 0.001) and there was a direct association with the disease (OR = 36.37). Moreover, only 1.12% of the patients in GCo presented cases of family history of breast cancer in first-degree relatives. This value is lower than the ones reported in the national literature. For instance, Paiva et al. (29), in a case-control study observed a family history of breast cancer in 12.5% of the patients with breast cancer and in 13.1% of the controls. Matos et al. (30) also showed the prevalence of the disease in 2.3% mother, 3.9% sister and 0.2% daughter affected by the disease.
Some studies have shown that the practice of physical exercise reduces the risk of breast cancer (31-33). In United Kingdon, 3.4% of post-menopausal breast cases in 2010 were associated with lack of exercise (34). Some possible mechanisms involved in breast cancer risk reduction are the reduction of hormone levels induced by exercise (35), body fat reduction (36,37), stimulation of antitumor immune activity and the reduction of insulin-like growth factors, which may increase cell division and inhibit cell death (35,37).
In our study, an inverse association was observed between the practice of moderate physical activity and breast cancer (p < 0.001). A greater number of GCo patients reported moderate physical activity practice (p = 0.001), while sedentary lifestyle was higher among the GCa. The moderate physical activity may have a protective effect against breast cancer according to the results.
The sedentary lifestyle is associated with 8% of breast cancer cases (38). Cohen et al. (39) in 2013 showed in white women that increased physical activity was associated with less chance of developing breast cancer, while sedentary lifestyle was associated with increased risk. The sedentarism is related with weigh gain, and cancer risk, including breast cancer, increases with increasing BMI (40). In a study with Japanese women, an inverse association with breast cancer was found among those who had the habit to walk more than an hour a day than those who walked less than an hour a day (33).
The benefits of practicing physical activity go beyond the disease prevention, also influencing the prognosis of breast cancer patients (41). Friedenreich et al. (42) observed that the practice of moderate to vigorous physical activity in post-menopausal women was enough to reduce the total body fat and adiposity. Therefore, this benefit may be related to the reduced risk of breast cancer in post-menopausal women.
Although there were a greater number of women practicing mild or moderate physical activity in the control group (p = 0.001), higher percentage of body fat (overweight or obese) was found in this group when compared to the GCa (p = 0.049).
The GCo patients had higher levels of 25(OH)D than the GCa patients (p = 0.008) (Table II). After stratification in deficient, insufficient, and sufficient vitamin D levels, the GCo group showed lower number of women with deficient levels and greater number of women with sufficient levels than GCa (p = 0.014) (Table II). Besides, an inverse association with breast cancer in the final model (OR = 0.95, 95% CI 0.91-0.99, p = 0.012) was observed (Table IV). Therefore, sufficient levels of vitamin D have given protective effect against breast cancer. Similar to our study, Park et al. (43) also dosed the vitamin D levels and stratified the women in groups by the levels of vitamin D, as deficient, insufficient, and sufficient (20 ng/dL, 20-29.9 ng/dL, and 30 ng/dL, respectively). It was assessed the levels of 25(OH)D in Korean women with breast cancer and in the general population, and found a direct association with breast cancer in women with deficient vitamin D serum levels when compared to those with sufficient levels, with no variation of menopausal status.
In Brazil, studies have shown insufficient levels of vitamin D in the elderly. Camargo et al. (2014) found concentration of 25(OH) D of 24.8 ng/mL in patients aged 67.9 ± 8.6 years (44). Arantes et al. (2013) demonstrated deficient levels (< 20 ng /mL) at 17% in women with age between 60 and 85 years old (45). Saraiva et al. (46) also found insufficient levels of 25(OH)D in 43.8% of outpatients and 71.2% of institutionalized elderly patients. Saraiva et al. (2005) found Vitamin D deficiency in 15.4% and insufficiency in 41.9% of patients aged 79.1 ± 5.9 years (47).
According to the Nurses' Health Study results, comparing the highest quintile with the lowest quintile of vitamin D in patients, there was a 30% breast cancer reduction risk (48), with even more impact in postmenopausal women aged over 60 years. The vitamin D action in the risk reduction of breast cancer could be explained by the inhibition and control of mammary cell growth demonstrated in animal model (49,50), by the antiproliferative action of 1,25 (OH)2D in tumor-derived endothelial cells, by modulating the cell cycle, and by affecting tumor cell signaling (51). Vitamin D also has action on the expression of genes involved in growth, differentiation and apoptosis (52). The overexpression of p73 is associated with induction of apoptosis in animals and in humans and can be influenced by calcitriol (52,53). The presence of 25-hydroxyvitamin D3 1-α-hydroxylase (CYP27B1) in mammalian cells indicate that local production of vitamin D would lead to a paracrine or autocrine action in mammary cells protection (54). In the present study, breast cancer patients with low vitamin D levels, would probably have less protection against malignant transformation and tumor growth.
The menopausal status seems to influence the association between vitamin D and breast cancer. However, there is no consensus in the literature. In a recent meta-analysis evaluating nine prospective studies, no association of risk reduction was found for breast cancer in pre-menopausal women, however, for post-menopausal women a decrease of risk, especially for 25(OH)D levels between 27 and 35 ng/mL was verified (55). The protective effect of adequate levels of this vitamin in preventing breast cancer among post-menopausal women was demonstrated by Crew et al. (56). Other studies have found benefit in reducing breast cancer risk in pre-menopausal women with normal weight, mainly with an intake of 5 µg/day of vitamin D (57,58). Abbas et al.(59) found an inverse association between breast cancer in pre-menopausal women and vitamin D levels, without interaction with BMI and family history of breast cancer, also demonstrated among Japanese women.
Fedirko et al. (60) also demonstrated an inverse association between circulating levels of vitamin D and breast cancer among pre-and post-menopausal Mexican women in a case-control study. This study had a greater number of menopausal women among the cases. The 25(OH)D3 average was 18.6 ng/mL in the cases and 21.9 ng/mL in controls. Furthermore, among the controls, 36% of women had deficient vitamin D levels (25(OH)D3 < 20 ng/mL) and 9% sufficient levels (> 30 ng/mL). Similarly, in this study, in GCa group, a greater number of menopausal women was observed. Among GCo patients, 14.29% had deficient levels and 34.59% sufficient levels of vitamin D (Table II).
The serum levels of calcium, phosphorus and PTH did not present difference between case and control groups, independent of vitamin D status and despite the vitamin D levels. The calcium and the vitamin D supplement intake may have a role in reduction of breast cancer risk (61-63), but more studies are needed to confirm this association.
The direct association was found in this study between diabetes mellitus and breast cancer in univariate analysis, but it did not remain after the final model analysis.
Conclusion
This case-control study demonstrated that menopause and family history of breast cancer were considered risk factors for breast cancer in women independent of the women age.
The serum level of vitamin D was considered protective factor for breast cancer. Despite the vitamin D levels found in women with breast cancer being lower than those of the control group, both averages were stratified as sufficient level. The vitamin D status is not yet used for breast cancer screening in women. However, this result suggests that vitamin D may have a positive impact as strategy of breast cancer prevention or reduction. Thus, an interesting strategy would be to screening women with insufficient and deficiency vitamin D status to provide supplementation of this vitamin. Another strategy could be the encouragement of physical activity practice for health improvement, since the practice of moderate to intense physical activity had a protective effect for breast cancer. Thus, our findings will contribute to fill an important gap in the scientific literature.
References
1. WHO. Breast cancer: prevention and control; 2014. [ Links ]
2. Rossi M, McLaughlin J, Lagiou P, Bosetti C, Talamini R, Lipworth L, et al. Vitamin D intake and breast cancer risk: a case-control study in Italy. Ann Oncol 2009;20:374-78. [ Links ]
3. Lacey J, Kreimer A, Buys S, Marcus P, Chang S-C, Leitzmann M, et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer 2009;9(1):84. [ Links ]
4. INCA, Instituto Nacional do Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância. Estimativa 2014: Incidência de Câncer no Brasil, Ministério da Saúde; 2014. [ Links ]
5. Anderson LN, Cotterchio M, Kirsh VA, Knight JA. Ultraviolet Sunlight Exposure During Adolescence and Adulthood and Breast Cancer Risk: A Population-based Case-Control Study Among Ontario Women. Am. J. Epidemiol 2011;174(3):293-304. [ Links ]
6. Grant W. Update on Evidence that Support a Role of Solar Ultraviolet-B Irradiance in Reducing Cancer Risk. Anticancer Agents Med Chem 2013;13(1):140-6. [ Links ]
7. Knight J, Lesosky M, Barnett H, Raboud J, Veith R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2007;16(3):422-29. [ Links ]
8. Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol 2011;347:6. [ Links ]
9. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008;88(2):491S-99S. [ Links ]
10. Paula FJA. A insuficiência óssea na doença renal crônica: papel do paratormônio. Arq Bras Endocrinol Metabol 2009;53(9):1059. [ Links ]
11. Paula FJ, Lanna CM, Shuhama T, Foss MC. Effect of metabolic control on parathyroid hormone secretion in diabetic patients. Braz J Med Biol Res 2001;34(9):7. [ Links ]
12. Pena GG, Maia YCP, Mendes MCS, Furtado WR, Machado-Coelho GLL, Freitas RN. Physical Activity Is Associated with Malignant and Benign Breast Diseases in Low-Income Brazilian Women. Nutr Cancer 2013;66(4):707-15. [ Links ]
13. Abranches M, Mendes M, Pena G, Maia Y, Ribeiro S, Franceschini S, et al. Antioxidant vitamins and cytokines are altered in breast cancer. Eur J Cancer Prev 2011;20(5):403-10. [ Links ]
14. Eberl MM, Fox CH, Edge SB, Carter CA, Mahoney MC. BI-RADS Classification for Management of Abnormal Mammograms. J Am Board Fam Med 2006;19(2):161-64. [ Links ]
15. WHO. WHO Expert Committee on Physical Status: the Use and Interpretation of Anthropometry (1993: Geneva, Switzerland). Physical status: the use of and interpretation of anthropometry. Geneva, World Health Organization, 1995. Available from: www.who.int/iris/handle/10665/37003. [ Links ]
16. WHO. Global Status report on alcohol-World Health Organization. Geneva; 2004; Available from: http://www.who.int/substance_abuse/publications/global_status_report_2004_overview.pdf. [ Links ]
17. CDC. Reducing the Health Cunsequences of Smoking: 25 Years of Progress. A Report of the Surgeon General Rockville, Maryland: Center for Chronic Disease Prevention and Health Promotion. Office on Smoking and Health; 1989. p. 89-8411. [ Links ]
18. CELAFISCS. Centro de Estudos do Laboratório de Aptidão Física de São Caetano do Sul; 2004. Available from: www.celafiscs.com.br. [ Links ]
19. American Cancer Society. What are the risk factors for breast cancer? 2015. Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-risk-factors. [ Links ]
20. Lippman ME, Krueger KA, Eckert S, Sashegyi A, Walls EL, Jamal S, et al. Indicators of Lifetime Estrogen Exposure: Effect on Breast Cancer Incidence and Interaction With Raloxifene Therapy in the Multiple Outcomes of Raloxifene Evaluation Study Participants. J Clin Oncol 2001;19(12):3111-16. [ Links ]
21. Cancer CGoHFiB. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13(11):1141-51. [ Links ]
22. WHO World Health Organization. Breast cancer: prevention and control. 2014. Available from: http://www.who.int/cancer/detection/breastcancer/en/index3.html (cited 2014 12/31/2014). [ Links ]
23. Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute; 2012 (cited 2015 09/01/2015); http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 11 SEER data submission, posted to the SEER web site, April 12. [ Links ]
24. Cutler W, Bürki R, Kolter J, Chambliss C, Friedmann E, Hart K. Invasive Breast Cancer Incidence in 2,305,427 Screened Asymptomatic Women: Estimated Long Term Outcomes during Menopause Using a Systematic Review. PLoS ONE 2015;10(6):e0128895. [ Links ]
25. Phipps AI, Buist DSM, Malone KE, Barlow WE, Porter PL, Kerlikowske K, et al. Family history of breast cancer in first-degree relatives and triple-negative breast cancer risk. Breast Cancer Res Treat 2010;126(3):671-78. [ Links ]
26. Bethea TN, Rosenberg L, Castro-Webb N, Lunetta KL, Sucheston-Campbell LE, Ruiz-Narváez EA, et al. Family History of Cancer in Relation to Breast Cancer Subtypes in African American Women. Cancer Epidemiol Biomarkers Prev 2016;25(2):366-73. [ Links ]
27. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and Heritable Factors in the Causation of Cancer - Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343(2):78-85. [ Links ]
28. Fernandes GC, Michelli RAD, Scapulatempo-Neto C, Palmero EI. Association of polymorphisms with a family history of cancer and the presence of germline mutations in the BRCA1/BRCA2 genes. Hered Cancer Clin Pract 2016;14:2. [ Links ]
29. Paiva CE, Ribeiro BS, Godinho AA, Meirelles RdSP, Silva EVGd, Marques GDA, et al. Fatores de Risco para Câncer de Mama em Juiz de Fora (MG): um estudo caso-controle. Rev Bras Cancerol 2002;48(2):231-37. [ Links ]
30. Matos J, Pelloso S, Carvalho M. Prevalence of risk factors for breast neoplasm in the city of Maringá, Paraná state, Brazil. Rev. Latino-Am. Enfermagem 2010;18(3):352-59. [ Links ]
31. Maruti S, Willett W, Feskanich D, Rosner B, Colditz G. A Prospective Study of Age-Specific Physical Activity and Premenopausal Breast Cancer. J Natl Cancer Inst 2008;100(10):728-37. [ Links ]
32. Eliassen A, Hankinson SE, Rosner B, Holmes MD, Willett WC. PHysical activity and risk of breast cancer among postmenopausal women. Arch Intern Med 2010;170(19):1758-64. [ Links ]
33. Suzuki S, Kojima M, Tokudome S, Mori M, Sakauchi F, Fujino Y, et al. Effect of Physical Activity on Breast Cancer Risk: Findings of the Japan Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev 2008;17(12):3396-401. [ Links ]
34. Parkin DM. Cancers attributable to inadequate physical exercise in the UK in 2010. Br J Cancer 2011;105(Suppl 2):S38-S41. [ Links ]
35. Rundle A. Molecular Epidemiology of Physical Activity and Cancer. Cancer Epidemiol Biomarkers Prev 2005;14(1):227-36. [ Links ]
36. Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med 2008;42(8):636-47. [ Links ]
37. Friedenreich CM, Orenstein MR. Physical Activity and Cancer Prevention: Etiologic Evidence and Biological Mechanisms. J Nutr 2002;132(11):3456S-64S. [ Links ]
38. Zhou Y, Zhao H, Peng C. Association of sedentary behavior with the risk of breast cancer in women: update meta-analysis of observational studies. Ann Epidemiol 2015;25(9):687-97. [ Links ]
39. Cohen SS, Matthews CE, Bradshaw PT, Lipworth L, Buchowski MS, Signorello LB, et al. Sedentary behavior, physical activity, and likelihood of breast cancer among black and white women: a report from the Southern Community Cohort Study. Cancer Prev. Res 2013;6(6):566-76. [ Links ]
40. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335(7630):1134. [ Links ]
41. Keegan THM, Milne RL, Andrulis IL, Chang E, Sangaramoorthy M, Phillips K-A, et al. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the breast cancer family registry. Breast Cancer Res Treat 2010;123(2):531-42. [ Links ]
42. Friedenreich CM, Neilson HK, O'Reilly R, et al. Effects of a high vs moderate volume of aerobic exercise on adiposity outcomes in postmenopausal women: A randomized clinical trial. JAMA Oncol 2015;1(6):766-76. [ Links ]
43. Park S, Lee D, Jeon J, Ryu J, Kim S, Kim J, et al. Serum 25-hydroxyvitamin D deficiency and increased risk of breast cancer among Korean women: a case-control study. Breast Cancer Res Treat 2015;152(1):147-54. [ Links ]
44. Camargo MBR, Kunii lS, Hayashi LF, Muszkat P, Anelli CG, Marin-Mio RV, et al. Modifiable factors of vitamin D status among a Brazilian osteoporotic population attended a public outpatient clinic. Arq Bras Endocrinol Metabol 2014;58:572-82. [ Links ]
45. Arantes HP, Kulak CAM, Fernandes CE, Zerbini C, Bandeira F, Barbosa IC, et al. Erratum to: Correlation between 25-hydroxyvitamin D levels and latitude in Brazilian postmenopausal women: from the Arzoxifene Generations Trial. Osteoporos Int 2013;24:2. [ Links ]
46. Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LMQ, Vieira JGH, Maeda SS, et al. Prevalência da deficiência, insuficiência de vitamina D e hiperparatiroidismo secundário em idosos institucionalizados e moradores na comunidade da cidade de São Paulo, Brasil. Arq Bras Endocrinol Metabol 2007;51:437-42. [ Links ]
47. Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LMQ, Vieira JGH, Kunii I, et al. Influence of ultraviolet radiation on the production of 25 hydroxyvitamin D in the elderly population in the city of São Paulo (23 o 34'S), Brazil. Osteoporos Int 2005;16(12):1649-54. [ Links ]
48. Giovannucci E. Vitamin D and Cancer Incidence in the Harvard Cohorts. Ann Epidemiol 2009;19(2):84-8. [ Links ]
49. Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol. Aspects Med 2008;29(6):388-96. [ Links ]
50. Gross M, Kost SB, Ennis B, Stumpf W, Kumar R. Effect of 1,25-dihydroxyvitamin D3 on mouse mammary tumor (GR) cells: Evidence for receptors, cellular uptake, inhibition of growth and alteration in morphology at physiologic concentrations of hormone. J. Bone Miner Res.1986;1(5):457-67. [ Links ]
51. Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL. Antiproliferative Effects of 1α, 25-Dihydroxyvitamin D3 and Vitamin D Analogs on Tumor-Derived Endothelial Cells. Endocrinology 2002;143(7):2508-14. [ Links ]
52. Mocellin S. Vitamin D and cancer: Deciphering the truth. Biochim Biophys Acta 2011;1816(2):172-78. [ Links ]
53. Ma Y, Yu W-D, Hershberger PA, Flynn G, Kong R-X, Trump DL, et al. 1α,25-Dihydroxyvitamin D3 potentiates cisplatin antitumor activity by p73 induction in a squamous cell carcinoma model. Mol. Cancer Ther 2008;7(9):3047-55. [ Links ]
54. Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol 2011;347(1-2):55-60. [ Links ]
55. Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma Vitamin D Levels, Menopause, and Risk of Breast Cancer: Dose-Response Meta-Analysis of Prospective Studies. Medicine 2013;92(3):123-31. DOI:10.1097/MD.0b013e3182943bc2. [ Links ]
56. Crew K, Shane E, Cremers S, McMahon D, Irani D, Hershman D. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol 2009;27(13):2151-56. [ Links ]
57. Abbas S, Linseisen J, Chang-Claude J. Dietary Vitamin D and Calcium Intake and Premenopausal Breast Cancer Risk in a German Case-Control Study. Nutr Cancer 2007;59(1):54-61. [ Links ]
58. Lee M-S, Huang Y-C, Wahlqvist ML, Wu T-Y, Chou Y-C, Wu M-H, et al. Vitamin D Decreases Risk of Breast Cancer in Premenopausal Women of Normal Weight in Subtropical Taiwan. J Epidemiol 2011;21(2):87-94. [ Links ]
59. Abbas S, Chang-Claude J, Linseisen J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Cancer 2009;124(1):250-55. [ Links ]
60. Fedirko V, Torres-Mejía G, Ortega-Olvera C, Biessy C, Ángeles-Llerenas A, Lazcano-Ponce E, et al. Serum 25-hydroxyvitamin D and risk of breast cancer: results of a large population-based case-control study in Mexican women. Cancer Causes Control 2012;23(7):1149-62. [ Links ]
61. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85(6):1586-91. [ Links ]
62. McCullough ML, Rodríguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, et al. Dairy, Calcium, and Vitamin D Intake and Postmenopausal Breast Cancer Risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomarkers Prev 2005;14(12):2898-904. [ Links ]
63. Lin J, Manson JE, Lee I, Cook NR, Buring JE, Zhang SM. INtakes of calcium and vitamin d and breast cancer risk in women. Arch Intern Med 2007;167(10):1050-59. [ Links ]
![]() Correspondence:
Correspondence:
Maria do Carmo Gouveia Peluzio.
Laboratório de Bioquímica Nutricional.
Departamento de Nutrição e Saúde.
Universidade Federal de Viçosa.
Av. Ph. Rolfs, s/n. Campus Universitário.
Viçosa, 36570-900. Minas Gerais, Brazil
e-mail: mcgpeluzio@gmail.com
Received: 01/10/2015
Accepted: 18/07/2016