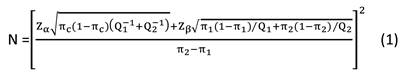INTRODUCTION
Obesity is characterized by an excessive accumulation of adipose tissue, which is associated with an increase in the number and size of adipocytes. Adipocytes are not only involved in storing triglycerides (TGs) as key regulators in energy homeostasis but are also involved in secreting multiple biologically active molecules, so-called adipokines, which link obesity to its associated complications, especially insulin resistance (IR) and type 2 diabetes. Notable among these adipokines, plasma adiponectin is decreased in obesity and associated complications 1,2; however, leptin contributes to obesity-associated IR 3-5. Dysregulated production or secretion of these adipokines caused by excess or dysfunctional adipose tissue may contribute to the development of obesity-related metabolic diseases.
Based on the complex interplay between adipokines, obesity is also characterized by a chronic low-grade inflammation with increased oxidative stress state 6,7. Oxidative stress is caused by increased free radical production. Cells' ability to detoxify radicals and to repair damaged molecules is impaired. Increased oxidative stress in obesity is associated with metabolic disorders and is suggested to participate in the onset and progression of these disease processes 8-11. Free radical production in oxidative stress may covalently modify membrane-associated or intracellular proteins, inducing a variety of cellular damage directly or indirectly through the production of a variety of membrane lipid peroxidation products. Principal among these is 4-hydroxynonenal (4-HNE), which is derived from the peroxidation of n-6 polyunsaturated fatty acids, such as arachidonic and linoleic acids. 4-HNE reacts with amino acids, such as cysteine, lysine and histidine, and forms stable adducts with proteins, thereby modulating the activities and expression of various proteins. As we have previously reported, at high concentrations, 4-HNE is cytotoxic to several cell types, whereas micromolar and submicromolar concentrations of 4-HNE have been shown to induce various nontoxic, cell-specific effects 2. Oxidative stress in adipose tissue plays an etiological role in a variety of obesity-related metabolic disorders. Using a high-fat diet-induced obesity mouse model, we reported that obesity was associated with increased adipose tissue 4-HNE formation 2,12. In both 3T3-L1 and primary mouse adipocytes, 4-HNE treatment at nontoxic concentrations decreased adiponectin secretion via an ubiquitin-proteasome regulated mechanism 2.
Obesity is associated with decreased plasma adiponectin concentrations and increased production of lipid peroxidation products in adipose tissue, including 4-HNE 13,14. There may be a relationship between the level of adipokines and 4-HNE. This study aimed to investigate the effects of 4-HNE accumulation on plasma adiponectin and leptin secretion in the Chinese non-diabetic obese (NDO) population. Plasma 4-HNE, adiponectin and leptin levels of 160 non-diabetic obese (NDO) patients and 160 healthy subjects were determined by ELISA, and their associations with adiposity, glucose, lipid profiles, insulin secretion and insulin sensitivity were studied.
MATERIALS AND METHODS
STUDY POPULATION
The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the local and regional ethics committees. All investigated patients gave their written informed consent to participate in the study. We enrolled 320 people who participated in a health check-up at the Department of Medicine Examination Center, the Second Affiliated Hospital of Harbin Medical University, China. There were 160 NDO subjects, and the others were non-diabetic non-obese control participants matched by age and gender. People with active liver or endocrine disease (including any type of diabetes mellitus), cardiovascular disease, renal impairment, malignancy, and alcohol or drug dependence were excluded. The study population was also limited to non-smoker, non-pregnant individuals free of clinically significant infectious diseases. Neither obese patients nor non-obese controls were taking lipid lowering, hypoglycemic, anti-inflammatory or antithrombotic medications or dietary supplements.
ANTHROPOMETRIC MEASUREMENTS
Anthropometric measurements of individuals wearing light clothing and without shoes were conducted by well-trained examiners. Height was measured to the nearest 0.1 cm with a portable stadiometer. Weight was measured in an upright position to the nearest 0.1 kg with a calibrated scale. BMI was calculated by dividing the weight (kg) by height squared (m2). Obesity was defined as BMI ≥ 27.5 kg m-2. Waist and hip circumferences (WC and HC) were measured at the level of the umbilicus and at the level of the maximum girth between the iliac crest and the crotch, respectively. Blood pressure (BP) was measured at the subjects' right hand with the subjects sitting after 5 min of rest using a calibrated sphygmomanometer (Hawksley, WA Baum Co, USA). All measurements of anthropometric indices and BP were performed by well-trained physicians, nurses or research staff. Each measurement was taken twice, and the average value was calculated. The homoeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = Glucose-Oral glucose tolerance test (OGTT) 0 min (mmol/L) × fasting insulin (µ IU/mL) / 22.5 15.
BIOCHEMICAL MEASUREMENTS
After an overnight fast of 10 h, venous blood samples were collected to measure glucose-OGTT 0 min, fasting insulin, blood lipids including total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) and liver function, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), hemoglobin A1C (HbA1C). Blood samples were also drawn 120 min after 75 g glucose load to measure glucose. Plasma glucose levels were measured using a hexokinase enzymatic method. Insulin was measured by a radioimmunoassay with human insulin as a standard (Linco, St Charles, MO). TG, TC, LDL-C, HDL-C and tests were performed enzymatically 16.
4-HNE AND ADIPOKINES MEASUREMENTS
The plasma concentrations of 4-HNE, adiponectin and leptin were measured by commercially available ELISA kits (Cusabio Life Science Inc., Wuhan, China, for 4-HNE measurements; RayBiotech, Norcross, UK for adiponectin and leptin determinations). 4-HNE ELISA kits were used to test the total endogenous 4-HNE bound to protein. The intra- and inter-assay coefficients of variations were < 8% and < 10% (4-HNE), < 10% and < 12% (adiponectin) and < 10% and < 12% (leptin), respectively. Measurements of the 4-HNE and adipokine levels in plasma were performed according to the recommendations of the manufacturers.
STATISTICAL ANALYSES
Data are expressed as the means ± SD for parameters with a normal distribution. Comparisons between groups (NDO vs. healthy controls) were analyzed by Student's unpaired t-tests for parameters with a normal distribution. Correlations between continuous variables were assessed by calculation of the linear regression using Pearson's test. p < 0.05 was considered statistically significant. We performed sample size estimation of the case-control study according to the formula 1 below.
Based our preliminary experiment, some parameters were estimated as π1 = 0.3, π2 = 0.6, πc = 0.5, Q1/Q2 = 1, α = 0.05, β = 0.10, and we obtained N ≈ 90. To better find some differences between groups, we increased the sample size to 160 for each group.
Based our preliminary experiment, some parameters were estimated as π1 = 0.3, π2 = 0.6, πc = 0.5, Q1/Q2 = 1, α = 0.05, β = 0.10, and we obtained N ≈ 90. To better find some differences between groups, we increased the sample size to 160 for each group.
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
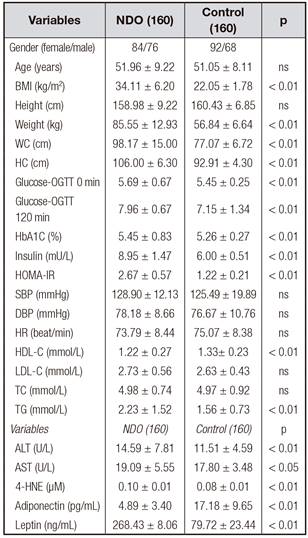
The characteristics of the study participants are shown in table I.
PLASMA 4-HNE LEVELS WERE INCREASED IN THE NDO POPULATION
Fasting plasma 4-HNE levels ranged from 0.09 to 0.11 µM, and the median was 0.10 ± 0.01 µM in the NDO population. The plasma concentrations of 4-HNE were significantly higher in the NDO subjects than in the control subjects (0.10 ± 0.01 µM vs. 0.08 ± 0.01 µM, NDO vs. control, p < 0.01) (Table I).
PLASMA ADIPONECTIN LEVELS WERE DECREASED WHEREAS PLASMA LEPTIN LEVELS WERE INCREASED IN THE NDO POPULATION
The fasting plasma adiponectin levels were more than 3 times lower in the NDO group compared with the healthy controls (4.89 ± 3.40 pg/mL vs. 17.18 ± 9.65 pg/mL, p < 0.01) (Table I). However, fasting plasma leptin levels were lower in the NDO group compared with healthy controls (268.43 ± 8.06 ng/mL vs. 79.72 ± 23.44 ng/mL, p < 0.01) (Table I).
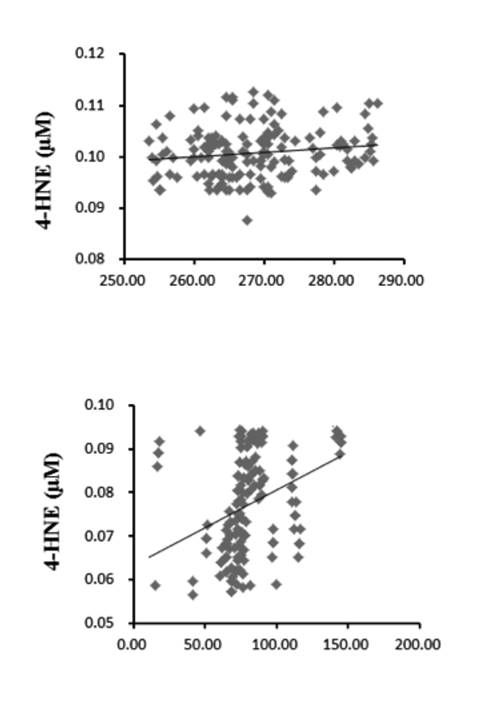
Correlation between plasma 4-hne and adiponectin/leptin levels In all subjects, the levels of fasting plasma 4-HNE were positively correlated with leptin and were negatively correlated with adiponectin (p < 0.01) (Table I, Fig. 1 and Fig. 2).

Figure 1 Association of 4-HNE with adiponectin. In NDO individuals (A) and healthy controls (B). The levels of fasting plasma 4-HNE were negatively correlate
4-HNE LEVELS ARE CLOSELY RELATED WITH GLUCOSE METABOLISM, INSULIN SECRETION AND SENSITIVITY
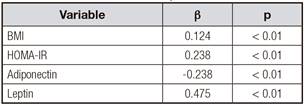
To further investigate the relationship between 4-HNE and other anthropometric parameters, multiple stepwise regression analysis involving all parameters, including fasting glucose, 2 h-glucose, fasting insulin, HbA1C, HOMA-IR, TC, TG, LDL-C and HDL-C, with significant correlations with plasma 4-HNE was performed. In all subjects, the levels of fasting plasma 4-HNE were positively correlated with BMI and HOMA-IR (p < 0.05) (Table II).
DISCUSSION
Oxidative stress is associated with obesity and IR and is considered to contribute to the progression toward obesity-related metabolic disorders. Recent evidence demonstrates that the imbalance between oxidative stress and antioxidant defense also triggers insulin resistance 6,17. As reported in 3T3-L1 adipocytes, 4-HNE treatment at nontoxic concentrations increased 4-HNE-insulin receptor substrate (IRS) adducts levels, leading to adipocyte IR 18. 4-HNE may play an important role in the pathogenic cellular changes that cause IR and other abnormalities in obesity, and 4-HNE may also mediate disease processes promoted by obesity 19. We and others have found that the levels of 4-HNE are higher in the blood and/or muscle tissue of obese individuals than in lean subjects 19. Previous studies indicated that 4-HNE inhibited adiponectin gene expression and secretion in 3T3-L1 adipocytes; however, the mechanisms were not investigated 20.
Strong evidence supports that oxidative stress plays a pathologic role in obesity-related disorders; however, the underlying mechanisms of the effects of 4-HNE on adiponectin gene expression and secretion, as well as its involvement in obesity-related decline, remain elusive. As reported 4-HNE, the most abundant lipid peroxidation end product, differentially regulates adiponectin gene expression and secretion by activating PPAR and accelerating ubiquitin-proteasome degradation 2. Although several animal experiments have suggested 4-HNE as an important regulator of glucose metabolism and insulin sensitivity, the clinical relevance of these findings in humans remains poorly characterized 18,20. The HNE-protein adduct ELISA is a method to detect HNE bound to proteins, which is considered as the most likely form of HNE in living systems 21. Although the detected absolute values of HNE-protein adducts were different, depending on the antibody used, both ELISA methods showed significantly higher values of HNE-protein adducts in the obese group 21. Intracellular HNE reacts rapidly with the thiol groups of glutathione and cysteine, with the -amino groups of lysine, and with the histidine residues of proteins 21. In this study, we used commercial ELISA kits to test the total endogenous 4-HNE bound to protein; our data showed that 4-HNE might be involved in the pathogenesis of non-diabetes obesity, as supported by two novel findings. First, the plasma 4-HNE concentrations were significantly increased in subjects with NDO compared with the age- and gender-matched healthy subjects, in contrast to the change pattern of circulating adiponectin levels. Second, the plasma 4-HNE levels were strongly associated with insulin sensitivity in both non-diabetes obese and healthy subjects.
Accumulated evidence suggests that adipose tissue oxidative stress plays a central and causal role in the pathogenesis of metabolic syndrome 8-11. Excessive fat accumulation increases the production of reactive oxygen species and lowers cellular antioxidant levels, leading to oxidative stress in adipose tissue. Various reactive oxygen species react with all cellular components; the hydroxyl radical-mediated peroxidation of polyunsaturated acyl chains of glycerophospholipids is particularly harmful because it results in the formation of lipid peroxidation production considered second messengers and the ultimate mediator of toxic effects elicited by oxidative stress. Of the lipid peroxidation products, 4-HNE is the most abundant and reactive aldehydic product derived from the peroxidation of n-6 polyunsaturated fatty acids 22-24. The results from our study are consistent with previous clinical observations and experimental investigations showing that obesity is associated with decreased plasma adiponectin concentrations and increased production of lipid peroxidation products in adipose tissue, including 4-HNE 13,14. A long-term high-fat diet led to obesity in mice, accompanied by decreasing the plasma adiponectin and increasing the adipose tissue 4-HNE content 2. Exposure of adipocytes to exogenous 4-HNE resulted in decreased adiponectin secretion in a dose-dependent manner, which was consistent with the significantly decreased intracellular adiponectin protein abundance 2. Polyunsaturated fatty acid (PUFA) in the membrane phospholipids or in circulating lipoproteins might be subjected to non-enzymatic, free radical-driven lipid peroxidation under certain pathological conditions, such as inflammation and obesity 25. Both types of lipid oxidation result in the formation of highly reactive lipid hydroperoxides, including proinflammatory leukotrienes. Small end-products of lipid peroxidation, such as 4-HNE and other aldehydes transported by activated granulocytes/monocytes, may facilitate and maintain generalized inflammation 25. The plasma adiponectin level is determined by complex intracellular regulatory mechanisms involved in gene expression, post-transcriptional/translational modification, and trafficking/secretion processes 26,27. Adiponectin is predominantly produced and secreted into circulation by adipocytes. Obesity-related plasma adiponectin decline is critically involved in the pathogenesis of obesity-related metabolic disorders. In contrast, 4-HNE exposure led to marked reductions in both intracellular adiponectin protein contents and secretion into media. The CHX-chase assay revealed that 4-HNE accelerated the intracellular adiponectin protein degradation rate by the ubiquitin-proteasome system. These data collectively suggest that 4-HNE can differentially regulate adiponectin gene expression and protein secretion in adipocytes, which may contribute to obesity-related plasma adiponectin decline 2.
Obesity represents a major health burden worldwide. White adipose tissue, especially in the visceral compartment, was recently discovered to be not just a simple energy depository tissue but also an active endocrine organ, releasing a variety of biologically active substances termed adipokines. Generally, adipokines play key roles in the regulation of glucose/lipid metabolism, insulin sensitivity and inflammation. Several adipokines are associated with obesity and have potential impact on obesity-related metabolic diseases. Multiple lines of evidence provide valuable insight into the roles of adipokines in the development of obesity and its metabolic complications.
BMI was correlated positively with leptin levels and negatively with adiponectin concentrations 6,28. By assessing the correlations between 4-HNE, as an oxidative stress product, and the formerly discovered classical adipokines, we found a significant negative correlation between the concentrations of adiponectin and 4-HNE. A significant positive correlation was also detected between leptin and the 4-HNE levels, which further supports the potential role of 4-HNE in the development of obesity-related disorders. Because adiponectin and leptin are considered to have opposing effects on inflammation in obesity 6,29, these findings indicate a pro-inflammatory role of 4-HNE in NDO patients, giving support to our study hypothesis.
There are several limitations of this study. For example, the sample size was relatively small. The correlation between 4-HNE and adipokines did not guarantee the existence of a causal relationship. Further prospective studies are required to determine whether elevated plasma 4-HNE is the enabling step of obesity or simply an accompanying or secondary response to obesity.