INTRODUCTION
Selenium is an essential trace element with antioxidant, immunomodulatory and anti-inflammatory activity 1 in the organism. It makes up the active site of glutathione peroxidase (GPx), an enzyme that acts against the common oxidative stress in critically ill patients.
Common complications in critically ill patients, such as systemic inflammatory response syndrome (SIRS), multiple organ failure and multiple organ dysfunction 1) (2 were associated with reduced plasma selenium levels and GPx. Indeed, plasma selenium and GPx were inversely correlated with the severity of disease and mortality and morbidity 1) (3. Some studies have shown that low selenium concentrations may also hinder wound healing 4) (5.
The oxidative stress and the inflammation process generated in the acute phase trigger a series of biochemical changes in the human body, increasing the nutritional demand of selenium, the deficiency of which could further aggravate the clinical condition. However, critically ill patients using total parenteral nutrition (PN) do not receive selenium because this mineral is not commonly offered in Brazil and others countries. Therefore, the evaluation of selenium levels is very important to treat or prevent such a deficiency in order to improve patient recovery. Although plasma selenium reflects the current selenium status and is the most widely used method for monitoring the levels of this mineral, its interpretation may be impaired during the inflammatory response 6. Thus, measurements of plasma selenium need to be carried along with parameters that reflect the inflammatory process. In clinical practice, some parameters that indicate the inflammatory response are: reduction of albumin, transthyretin and HDL cholesterol, and a concomitant increase in acute phase protein such as C-reactive protein (CRP) 7) (8) (9) (10) (11 .
According to a recent study, transthyretin may reflect the selenium intake and could be considered a biomarker 12. The positive correlation of plasma selenium and transthyretin was reported among septic patients, but was not found in patients with systemic inflammatory response syndrome 13. Considering that, this issue is still little explored in the literature. This study aims to investigate the correlation of transthyretin with the plasma selenium of critically ill patients receiving PN.
MATERIAL AND METHODS
STUDY FEATURES
Prospective cohort study with 44 critically ill patients using PN. Blood samples were carried out in 3 stages (initial, 7th and 14th day of PN). Inclusion criteria were: hospitalization in the intensive care unit (ICU), use of total PN or PN as a primary source of nutrition and signature on the Free and Clarified Consent Form by patient or their guardian. This study was approved by the Research Ethics Committee of the School of Medical Sciences of State University of Campinas - UNICAMP (N. 538/2011). Exclusion criteria were: patients fed only oral and/or enteral nutrition and patients who had left the ICU before the first 72 hours of PN.
INDICATION AND PRESCRIPTION OF PARENTERAL NUTRITION
PN was prescribed by the physician responsible for the patient and by nutritional support team according to the European Society for Parenteral and Enteral Nutrition -ESPEN 15, and American Society for Parenteral and Enteral Nutrition -ASPEN 16. Since PN solutions have no selenium, the survey participants did not receive this mineral.
NUTRITIONAL ASSESSMENT STATUS
Anthropometry was performed with measurements of weight and height to calculate the body mass index (BMI), according to Lohman, Roche and Martorell 17 and the World Heatlh Organization 18. In case of confinement in bed, we opted for the method of estimating the weight 19) (20 and height according to their half arm span (Mitchell and Lipschitz) 21. In case of edema, the recommendation made by Duarte and Castellani 22 was used (subtracted 1 kg when edema was only on the ankle, 3-4 kg when it was on the knee, 5-8 kg when it was on the thigh and 10-12 kg when the edema was widespread). An inextensible and inelastic measuring tape of 100 cm and 0.1 cm accuracy, a Lange Skinfold Caliper(r) adipometer and a stadiometer coupled to the digital scale Líder(r) (2 kg to 300 kg capacity) were used.
LABORATORY EVALUATION OF THE CLINICAL CONDITION
To evaluate the clinical condition and inflammatory response, standardized routine tests were performed, examining the following compounds: albumin (colorimetric -bromocresol green), C-reactive protein (CRP) (nephelometry), transthyretin (nephelometry), HDL cholesterol (enzymatic -direct colorimetric) and creatinine (kinetic Jaffé colorimetric method with compensation). The measurements were made by the specialized team of the Clinical Pathology Laboratory of Hospital das Clínicas at UNICAMP.
ASSESSMENT OF SELENIUM STATUS
To measure GPx (whole blood), a RANSEL kit (RS504)(r) and a RANSEL CONTROL kit (SC692)(r) from the Randox Laboratory (San Franscisco, USA) were used. This technique is based on the method proposed by Paglia and Valentine 23. We collected 1 ml of blood in a heparinized bottle, stored at -80 °C. Subsequently, 0.05 ml heparinized whole blood was diluted with 1 ml diluent agent, and incubated for 5 minutes and 1 ml of Drabkin's hemolyzing reagent was added. After mixing the samples, the tests were started. The RANSEL RX Daytona equipment at 340nm was used to read the samples, and the normal range of GPx (whole blood) was from 4171 to 10881 U/l. The procedure and the reading of the samples were performed by the Laboratory of Exercise Biochemistry in the Biology Institute - UNICAMP.
To dose plasma selenium, blood was collected in dry tubes (free of trace elements) and centrifuged to separate the plasma. The samples were stored at -20 °C until the time of analysis. Plasma samples were digested in pyrex glass tubes (by wet acid). After the addition of 5 ml of nitric acid 68% P.A. (Merck), the samples were kept at rest overnight. Thereafter, digestion occurred in the digestion block with an initial temperature of 50 °C, which was gradually increased until reaching a maximum of 150 °C. The purpose of this step was to eliminate organic substances and reduce selenium in the solution into selenium IV. In the next step 5 mL HCl 1.2N was added and the samples were heated for two more hours (at 100 °C). Subsequently, the solutions were diluted with deionized water to 25 mL. Selenium reading occurred through the method of atomic absorption spectrometry by generation of hydrides coupled to the quartz cell (HGQTAAS) (model Z5000, Hitachi, Tokyo, Japan) 24),(25),(26). The normal range for plasma selenium concentration was between 60-120 μg/L 27) (28. The procedure and sample readings were made in the Nutrition Minerals Laboratory - from the School of Pharmaceutical Sciences, University of São Paulo - USP.
All materials used (glassworks, tips and plastics) had nitric acid bath of 30% for at least 12 hours, and were rinsed 10 consecutive times with deionized water for demineralization.
SEVERITY ASSESSMENT
To evaluate the severity, the score of the Acute Physiologic and Chronic Health Evaluation -APACHE II 29- was used as well as the Sequential Organ Failure Assessment -SOFA 30.
STATISTICAL ANALYSIS
The data statistical treatments were made through the SAS System for Windows (Statistical Analysis System), version 9.4. SAS Institute Inc, Cary, NC, USA. Exploratory data analysis was made through summary measures (frequency, percentage, mean, standard deviation, minimum, median, and maximum). A comparison between the times and between the groups over time was performed with the ANOVA test for repeated measures with the response variables processed in ranks. Mann-Whitney test was used for comparison between groups. The correlation between the amounts of plasma selenium with numerical variables was assessed using Spearman's coefficient. The significance level was 5%.
RESULTS
The sample consisted of 44 critically ill patients with a mean age of 58.9 ± 14 years that had PN primarily for medical reasons. The primary diagnosis of patients were: gastrointestinal cancer, sepsis, trauma, acute abdomen, inflammatory, inflammatory bowel disease and pancreatitis. Table I shows the clinical characterization of the sample.
Table I Sample description according to gender, age, PN indication, nutritional status and final outcome
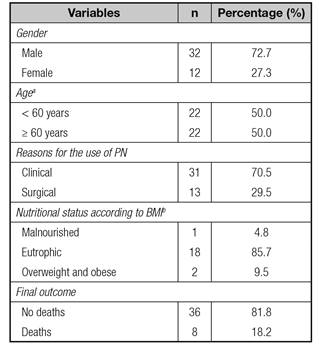
BMI: body mass index: aIn Brazil, over 60 years-old is considered elderly; bIt was possible to measure and/or estimate weight and height of only conscious patients without generalized edema (n = 21).
Concerning evaluation of severity, the mean and standard deviation of APACHE II and SOFA were 14.9 ± 5.7 (8.0-26.0 range) and 5.7 ± 2.8 (2.0-10.0 range), respectively.
At the beginning of the study, all patients had elevated CRP levels and low selenium levels (100%). Creatinine was high (29.5%) and GPx was below normal in 50% of the evaluated cases. HDL cholesterol (85.3%), albumin (97.3%) and transthyretin (97.1%) were low in most of them. Table II shows the evolution of biochemical indicators over the three assessments.
Table II Evolution of biochemical indicators of nutritional status and inflammatory profile over the three assessments

CRP: C-reactive protein; GPx: glutathione peroxidase (whole blood); aStatistically significant increase of transthyretin levels between the first and last dosing (p = 0.05) - ANOVA for repeated measures with the response variables processed into ranks.
In the first evaluation, there was positive correlation of selenium levels with the GPx (r = 0.46; p = 0.03). Throughout two weeks, there was a positive correlation between plasma selenium with transthyretin (r = 0.71; p = 0.05) and creatinine (r = 0.67; p = 0.03) (Table III, Fig. 1). There was no correlation of transthyretin with creatinine (r = 0.42; p = 0.26).
Table III Correlations of plasma selenium with the other markers
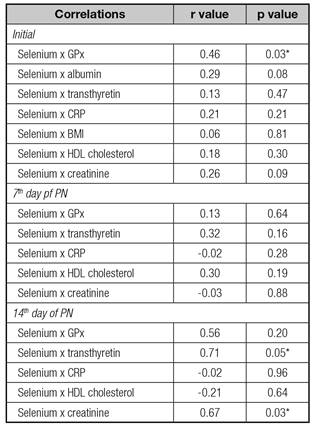
GPx: glutathione peroxidase (whole blood); CRP: C-reactive protein; BMI: body mass index. *p < 0.05 - Spearman's correlation coefficient.

Figure 1 Scatter plots showing correlations of bivariate plasma selenium with transthyretin and creatinine.
Regarding mortality, there was no statistical difference in selenium and GPx levels between the death group and the non-death group (p > 0.05). On the 7th day (2nd evaluation), the death group had a mean selenium concentration of 17.8 ± 4.4 μg/L and the non-death group of 24.9 ± 10.7 μg/L, a trend toward significance (p = 0.09) was observed. However, there was no statistically significant difference at any time of the evaluation.
DISCUSSION
Selenium is a trace element with antioxidant, immunomodulatory and anti-inflammatory activity, but critically ill patients using total parenteral nutrition (PN) do not receive selenium because this mineral is not commonly offered. Thus, the evaluation of selenium levels is very important to treat or prevent the deficiency. Recent studies have shown that transthyretin may reflect the selenium intake and could be considered a biomarker 6) (12) (13) (14. However, this issue is still little explored in the literature. Therefore, this study aimed to investigate the correlation of transthyretin with the plasma selenium of critically ill patients receiving PN.
Mahn, Toledo and Ruz 12 suggest that transthyretin can be a biomarker of bioactive state of selenium because it responded to supplementation (SeMSeCys) offered to a group of rats that had no inflammatory process. In the study of Brodska et al., 13, there was no correlation of plasma selenium with transthyretin in patients with SIRS, however, in septic patients, selenium was correlated with transthyretin both in the supplemented group and the non-supplemented group.
In the study, the plasma selenium was below the referenced level in all patients since the first assessment. Along with this improvement (by the 14th day of evaluation), a strong positive correlation between thransthyretin and plasma selenium was observed.
It is known that, in critically ill patients, transthyretin seems to reflect the inflammatory process more than the nutritional status 32) (33. Nevertheless, we did not find a negative correlation of transthyretin and of plasma selenium with CRP, which is contrary to the results observed in the Blass et al.´s 5 study.
In relation to the positive correlation of selenium with creatinine, renal failure causes selenium accumulation due to the difficulty of excretion 34. Thus, continuously low selenium levels could still be overestimated in patients with elevated creatinine. We also know that elevated levels of creatinine can influence transthyretin levels, however there was no positive correlation between transthyretin and creatinine observed in our study. Also, the strong positive correlation of transthyretin with plasma selenium, detected during the 2nd week of assessment, demonstrated that transthyretin may reflect selenium plasma. This correlation was verified after the acute inflammatory phase of the patient passed.
With regard to low levels of selenium and GPx, a positive correlation was found between the two, as observed in another study 13. GPx is a selenium-dependent enzyme that reflects the status of this mineral. It is also responsible for approximately 30% of plasma selenium measured 35) (36. It is known that plasma selenium and GPx may be altered in critically ill patients due to oxidative stress 35.
Forceville et al., 37 found selenium values in patients with SIRS lower than in patients without SIRS. Manzanares et al., 38 demonstrated that the patients in critical condition without SIRS (and APACHE, less than 9) had selenium levels similar to the healthy patients group (mean and standard deviation = 72.8 ± 13.1 μg/L). Heyland et al. 39 observed that the initial plasma selenium was within normal limits in North American patients with multiple organ failure, and found no difference in the levels of selenium between the group with sepsis and the control group. It seems that in studies with critically ill patients conducted in places with selenium rich soil (as in the U.S.A.) there are no such low levels of selenium as those studies conducted in places where the soil is poor in selenium (regions of Europe and South America) 39) (40. Thus, it is not yet clear whether the plasma selenium concentrations reflect the inflammatory process and/or mineral nutritional deficiencies 40.
Our study was conducted in the state of Sao Paulo (southeast Brazil), a region considered to have selenium poor soil 41) (42, and actually the plasma selenium values found were similar or lower to those found in other studies 5) (31) (38) (43. It is possible that the inflammatory response did contribute to low selenium levels, however, there was no correlation of selenium with CRP at any time. Therefore, it is presumed that the reduced levels of selenium and GPx are the consequence of both the inflammatory response as well as the intake/insufficient infusion of this trace element.
Selenium supplementation for patients using PN is recommended by ASPEN 44 (60-100 μg/day or 400 μg/day in severe cases), but it is not a practice commonly performed in Brazil and in other countries (developed countries and developing country). We know this is one of the first studies assessing the correlation of transthyretin with plasma selenium in critically ill patients using total PN or PN as the main source of nutrition. Thus, conducting randomized clinical trials is essential to confirm the findings of our study, mainly because research with critically ill patients using PN with and without selenium is scarce.
With regard to mortality, we observed a trend of lower selenium levels in patients who died. It is possible that the assessment period (14 days) and/or the number of participants may not have been sufficient for the statistical difference to be evident. Costa et al., 31, also found, with a sample of 110 patients, no statistical difference in plasma selenium among the deceased and survivor groups. However, studies have reported a reduced risk of mortality among patients supplemented with high doses of selenium 45. In fact, the amount, the time to start and the supplementation time are still discussed, but the importance of supplementation is emphasized since this seems to prevent progression or contribute to the treatment of diseases and complications associated with these disabilities.
The main limitation of this study concerns the sample loss which occurred due to death or withdrawal of PN before the 14th day of assessment. In addition, the complexity and heterogeneity of the sample due to illness, and various clinical complications may have underestimated or overestimated selenium levels. However, both the high mortality rate and the heterogeneity of the sample are features commonly found among patients followed in the ICU.
FINAL REMARKS
Due to a positive correlation observed after the acute inflammatory phase in patients, we suggest that transthyretin may reflect plasma selenium levels. Therefore, the lower levels of it detected at the start of PN show the need for selenium monitoring and supplementation, especially in patients with low transthyretin.
STATEMENT OF AUTHORSHIP
Contributed to conception or design: Renata Germano Borges de Oliveira Nascimento Freitas; Roberto José Negrão Nogueira; Gabriel Hessel. Contributed to acquisition, analysis, or interpretation: Renata Germano Borges de Oliveira Nascimento Freitas; José Negrão Nogueira; Gabriel Hessel. Drafted the manuscript: Renata Germano Borges de Oliveira Nascimento Freitas. Critically revised the manuscript: Renata Germano Borges de Oliveira Nascimento Freitas; Roberto José Negrão Nogueira; Silvia Maria Franciscato Cozzolino; Ana Carolina Junqueira Vasques; Matthew Thomas Ferreira; Gabriel Hessel. Gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy: Renata Germano Borges de Oliveira Nascimento Freitas; Roberto José Negrão Nogueira; Silvia Maria Franciscato Cozzolino; Ana Carolina Junqueira Vasques; Matthew Thomas Ferreira; Gabriel Hessel.












 Curriculum ScienTI
Curriculum ScienTI

