INTRODUCTION
The only source of nutrients for growth of the fetus is maternal blood 1. Iron is vital for early brain growth and function because it supports neuronal and glial energy metabolism, neurotransmitter synthesis, myelination, and development of red blood cells, blood vessels and muscles 2,3. As fetal iron results exclusively from the mother by active transport function of the placenta, the amount transferred could be reasonably assumed to be influenced by the amount of maternal iron available 4. Iron transfers from the mother to the fetus are regulated by the placenta and involve placental structure, iron transporters, and regulation of placental expression of these proteins 5.
The last trimester of pregnancy is the period of the most important weight gain and iron storage in the fetus. Amounts of iron in low birth children are lower than full-term newborns and after birth accumulated iron compared to full-term newborns. After birth, reserves acquired during pregnancy will be used during the first 4-6 months of life 6.
Iron deficiency anemia is the most common preventable nutritional deficiency during pregnancy, which have an impact on morbidity and maternal as well as perinatal complications, such as premature delivery, intrauterine growth retardation, and perinatal death 7) (1. In Blida, north Algeria, prevalence of iron deficiency anemia during pregnancy in 2006 was 46.66% 8.
Published studies highlighted the relationship between the maternal and neonatal iron status, but the results remained discrepant 9) (10. Challenges and concerns with routine blood sampling among healthy newborns still encountered. Studies were done to compile reference data on iron status among healthy newborns using both venous and cord blood 11),(12. Understanding the relationship between maternal and fetal iron status may help exert efforts to prevent iron deficiency in pregnancy and infancy, and improve outcomes for mothers and infants.
The present study was undertaken to find the possible relationships between maternal blood parameters which determine the iron status (hemoglobin [Hb], hematocrit [Hct], serum iron, total iron binding capacity of transferrin [TIBC], saturation coefficient of transferrin [CS] and serum ferritin) and blood from the umbilical cord vein, reflecting the placental exchange.
MATERIALS AND METHODS
The study was carried out on 97 couples of mothers and newborns scheduled to give birth by prophylactic cesarean in the Central Maternity Doctor Khaldi Abed Elazziz of Tébessa (east of Algeria) between January and August 2014.
In all these cases, pure prophylactic cesarean were performed after 37 to 42 weeks of gestation in women with a pregnancy of normal development and exhibiting no significant pathology which affected the metabolism of iron in the body. These women were aged between 22 and 42 years old. Primigravida or multigravida and indications of cesarean were conditioned by a notion of prior cesarean, contracted pelvis, breech present position.
For each selected woman, we explained the purpose and the experimental protocol approved by the Ethics Committee of the University Frères Mentouri Constantine 1. All women included in this study signed informed consent.
BLOOD SAMPLES COLLECTION
Under normal operating conditions at the central maternity of Tébessa, maternal arterial samples were prohibited; maternal blood samples were then collected from antecubital vein of the arm and without tourniquet before anesthesia.
Five milliliters were collected by a sterile 5 ml syringe. Two milliliters were put in an EDTA-K3 tube (Ethylene Diamine Tetra-Acetic) for the measurement of hematocrit (Hct) and hemoglobin (Hb), and three milliliters were placed in a dry tube for the determination of serum iron, serum ferritin and total iron binding capacity of transferrin (TIBC).
The blood of the umbilical vein was collected after two minutes of umbilical cord clamping, as soon as the newborn was removed and the umbilical cord was cut at the side of the placenta; the same quantities of blood were taken for the same analysis.
The fetal and maternal blood sample tubes were placed in a cooler and transported immediately to the laboratory for analysis.
Dosages of hemoglobin and hematocrit were performed immediately on the blood. Serum was recovered after centrifugation at 1,500 rpm for 15 minutes and stored in the refrigerator in the maternity laboratory at 4 °C. After blood sampling of all subjects of the day, samples were transported in a cooler to the laboratory of the Bouguera Boulaaras Bekaria Hospital for measuring other parameters as soon as samples were received.
BIOASSAYS
The rates of hemoglobin and hematocrit were determined on an automated counter Nihon Kohden type for hematological analysis (model MEK-6400K, Nihon Kohden Corporation, Tokyo, Japan). The serum iron was estimated by a colorimetric method using ferrozine as chromogen. The total iron binding capacity of transferrin was evaluated after transferrin saturation by an iron solution and adsorption of the excess over magnesium hydroxycarbonate (Fer Ferrozine + TIBC kit, ref.: 200643, Biomaghreb, Tunisia). The saturation coefficient of transferrin was calculated from the assay of serum iron and TIBC, CS% = ([serum iron/TIBC] × 100), serum ferritin was estimated by ELFA technology (Enzyme Linked Fluorescent Assay) using a Mini VIDAS automate (Ferritine kit, ref.: 30411, Biomerieux S.A., France).
STATISTICAL ANALYSIS
Statistical analyses were performed using XL STAT version 2009.1.01 (Addinsoft 1995-2009, USA). The results of this study are presented using descriptive statistics such as arithmetic mean, standard deviation and frequency. Analyses of relations between maternal and newborn blood parameters excluded all pairs of diabetic cases to avoid interference with their interpretation regarding iron status. The assumption of normality of data was verified by the Anderson-Darling test to use the statistical methods. Comparisons between two means were tested by t-test. Pearson's correlation was used to investigate possible association between variables. The significance level adopted was 5%.
RESULTS
AGE AND GESTITY
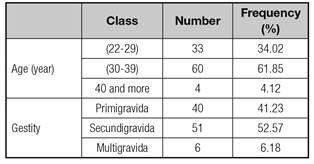
Our study group consisted of 97 women aged between 22 and 42 years old; the average age was 31.7 ± 4.7 years. The majority of pregnant women (61.85%) belonged to the age group of 30-39 years old. Depending on gestity, 40 women (41.23%) were primigravida, 51 (52.57%) were secundigravida, and six (6.18%) were multigravida (Table I).
No significant relationship (p > 0.05) was found between maternal iron status and age and gestity.
BLOOD PARAMETERS AND RELATIONSHIPS
In table II we presented the blood parameters, measured in the antecubital vein and umbilical vein, as mean ± standard deviation (SD) and extreme values. We noticed that maternal and fetal values generally fluctuated in the same way as evidenced by the similarity of standard deviations; the same remark was noticed for extreme values.
Table II Blood parameters in antecubital and umbilical veins (n = 97)

Hb: Hemoglobin; Hct: Hematocrit; TIBC: Total iron binding capacity of transferrin; CS: Saturation coefficient of transferrin.
Umbilical vein values were greater than those of maternal vein; serum iron and ferritin were, respectively, two and nine times greater in newborns than in mothers.
Except for serum iron, significant linear correlations were observed between fetal hemoglobin and other fetal parameters. Respectively, Hct and TIBC were positively correlated to Hb (r = 0.6, p < 0.0001 and r = 0.2, p = 0.04). CS and serum ferritin were negatively correlated to Hb (r = 0.28, p = 0.005 and r = 0.38, p < 0.0001) (Table III).
Table III Relationships between blood parameters (y) and hemoglobin (x) in the umbilical vein (n = 97)
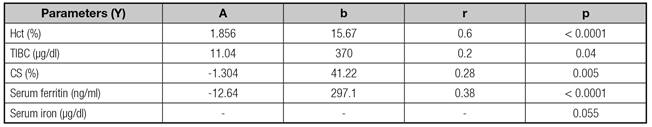
Hct: Hematocrit; TIBC: Total iron binding capacity of transferrin; CS: Saturation coefficient of transferrin; a: Regression coefficient; b: Constant term; r: Pearson's correlation coefficient.
Table IV shows correlations between Hb and other maternal parameters. Hb was only correlated positively with Hct (r = 0.79, p < 0.0001), and was negatively correlated with serum ferritin (r = 0.33, p = 0.001).
Table IV Relationships between blood parameters (y) and hemoglobin (x) in antecubital vein (n = 97)

Hct: Hematocrit; TIBC: Total iron binding capacity of transferrin; CS: Saturation coefficient of transferrin; a: Regression coefficient; b: Constant term; r: Pearson's correlation coefficient.
The relationships between maternal parameters and those of the umbilical vein are outlined in table V. Except for serum ferritin, fetal and mother parameters were positively correlated. The greatest correlation was seen between fetal and maternal serum iron (r = 0.39, p < 0.0001).
Table V Relationships between umbilical vein (y) and maternal (x) parameters (n = 97)
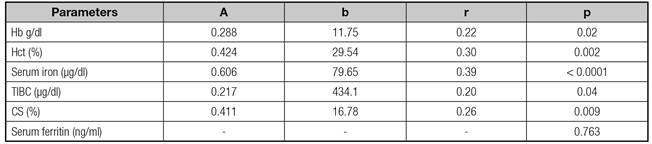
Hb: Hemoglobin; Hct: Hematocrit; TIBC: Total iron binding capacity of transferrin; CS: Saturation coefficient of transferrin; a: Regression coefficient; b: Constant term; r: Pearson's correlation coefficient.
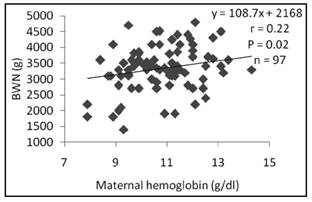
Birth weight (Fig. 1 and Fig. 2) was significantly related to maternal Hb (r = 0.22, p = 0.02) and Hct (r = 0.2, p = 0.004).
DISCUSSION
In this study, no blood parameter of iron status was correlated with maternal age or maternal gestity; age and multiple gestity did not seem to be risk factors for iron deficiency. There were discrepancies between authors. Chandyo et al. 13 reported women with parity ≥ two had higher mean hemoglobin concentration than nulliparous ones. In others studies on risk factors of maternal anemia during pregnancy, Emamghorashi et al. 14 and Veghari et al. 15 found that multiparity, but not age, influenced maternal iron status. Multiparity and short birth interval (< two years) between pregnancies created a large demand for iron, which was needed to develop the fetus and placenta.
The measured iron status parameters were higher in fetal blood than in maternal blood.
This finding was also noticed by Dop et al. 16 and Sa et al. 17 in their studies on anemia in pregnancy in Togo and Brazil respectively. The relatively low values we observed in Hb and Hct in maternal blood might be due to the plasma volume expansion resulting in hemodilution during pregnancy 18,19. However, the rates of hemoglobin and hematocrit were high in infants because of the increase in the number and size of red blood cells in cord blood 20. In addition, physiological change during pregnancy altered the composition of blood, amplified the transfer of certain hematopoietic micronutrients and increased the use of some others 19.
The negative link in the umbilical vein between Hb and ferritin and with the CS suggested a competition between hemoglobin-synthesis and storage of iron as ferritin, as it was reported by Macphail et al. 21, who studied the relationship between the iron status of 103 mothers and their newborns. The TIBC varied in the same way as Hb, which complied with the role of transferrin in the supply of iron to hemoglobin. These findings were in accordance with the study of Doc et al. 16.
The large variation of the fetal iron concentration (53-191 µg/dl) did not greatly affect the level of fetal hemoglobin, with variations remaining relatively low (11-19 g/dl). Similar results were reported by Singla et al. 22, who evaluated the parameters status of maternal and fetal iron and morphology of the placenta from 69 mothers and newborns in India. We believe that a link could be observed with iron concentrations below a certain critical threshold, not encountered in our study group, which exposed the newborn to diseases due to a strong iron deficiency.
Our results indicate a significant relationship between the concentration of Hb in the umbilical vein and the antecubital vein (r = 0.22, p = 0.02). This was consistent with Shoa et al. 2, who evaluated the relationship between iron status of mother and full term neonate to term and reported a high significance (r = 0.10; p ≤ 0.0001; n = 2775). However, Turkey et al. 23 did not a find a correlation between maternal and newborn hemoglobin. Thus, the maternal Hb affected the level of fetal Hb; as a result, a decrease in maternal Hb was likely to develop anemia in the newborn at an early age, poor cognitive and neurological development and risk of developing chronic diseases in adulthood, such as heart disease and type 2 diabetes 24,25.
In this study, significant correlations were found between newborns serum iron, TIBC and CS and those of their mothers. Adariana et al. 26 reported similar correlations. The feto-maternal relationship for serum iron was greater than other parameters (r = 0.39; p < 0.0001), and the fetal serum iron level was generally twice that of the mother. We deduced from this relationship that the fetus took over 60% of maternal iron, which passed in a counter-gradient way. A minimum iron concentration of 80 µg/dl was kept constant in the fetus, even if the mother was deficient in iron. This was possible by a regulation of iron transport proteins in the placenta ensuring an adequate supply of iron to fetal growth, even in mothers suffering from iron deficiency 27. Then the weak correlations shown between newborn and maternal parameters could not predict hematological and biochemical parameters of newborn of mildly or moderately anemic pregnant women 26.
Similarity to Shoa et al. 2 and Sa et al. 17, we observed a low rate of serum ferritin in mothers (12.37 ± 9.58) compared to newborns (109.64 ± 58.76). According to Gambling et al. 28, the concentrations of ferritin decreased significantly during pregnancy and the process was mediated by signals from the fetus whose nature was not known yet. On the other hand, like others 26)(29, we did not find any feto-maternal relationship for serum ferritin. However Shoa et al. 2 reported significant weak correlations (r = 0.07) due probably to their large sample. Iron transmitted to the fetus came from maternal stores as ferritin. The release of iron from ferritin is still being studied and the underlying mechanism is not clearly established yet 5.
From experiments on rats, which were widely used as a model for the human placenta function, a hierarchy of use of maternal iron was observed: the fetus was a priority, maternal hematocrit came on, and maternal iron stores were the last. This had serious consequences for the mother, if the observations obtained in rats had to be transposed to humans, because the mother needed iron stores not only for herself but also for breastfeeding as well as for future pregnancies 5) (30.
In this study of women with gestation in normal development, birth weight had linear relationship with maternal Hb and Hct (Fig. 1 and 2). Similar results were reported by Singla et al. 22, who also suggested a negative effect of anemia during pregnancy on different anthropometric measurements at birth. However, two studies on two different populations (US and UK) had not reported a relationship between markers of iron status (hemoglobin and mean corpuscular volume) at different stages of pregnancy and fetal growth 31) (32. In contrast, another study showed that the babies of iron-deficient anemia mothers had greater head circumference and were heavier than those from non-anemic non-iron-deficient mothers 14.
The effect of maternal anemia on intra-uterine growth is attributed to chronic deprivation of oxygen to the developing fetus 33. According to Rodríguez et al. 34, various biological mechanisms had been proposed to explain the ways in which iron deficiency, including in its most severe form, may adversely affect fetal growth. Maternal and fetal stress could result from increasing concentrations of noradrenaline generated by iron deficiency or hypoxia resulting from anemia. Excess of noradrenaline rises vagal activity, hence hyperinsulinemia, which may result in fetal weight increase.
Maternal and fetal stress, in turn, activated the production of the corticotropin-releasing hormone that stimulated fetal cortisol. Accordingly, the longitudinal growth of the fetus might be affected by the action of cortisol 35. Animal studies supported this hypothesis. The stress caused by iron deficiency was shown by the increase in cortisol levels due to iron-free diet in rats 36) (37)(38. Another plausible hypothesis of the effect of iron deficiency on fetal growth was that erythrocytes and the fetal-placental unit could suffer oxidative damage caused by iron deficiency. Finally, possible maternal infections could increase with iron deficiency, and might enable the production of the corticotropin-releasing hormone 35.
CONCLUSION
In the present study, we observed that age and gestity were not a risk factor for iron deficiency in pregnant women.
Hemoglobin, hematocrit, serum iron, total iron binding capacity of transferrin and saturation coefficient of transferrin in the fetus were in a linear relationship with those in the mother. Accordingly, any deficiency in these components of the status of iron or alteration in their metabolic regulation was likely to develop pathologies related to the optimal intake of iron in the newborn which could be complicated in adulthood.
Birth weight was affected by the rate of hemoglobin and maternal hematocrit. The other parameters of iron status did not seem to have much direct link with birth weight of newborns in the present study.
Much work has to be achieved with a larger population, depicting situations of high iron deficiency or excess for a better definition of the fetal-maternal relationships that may occur.