INTRODUCTION
Liver fibrosis is characterized by excessive accumulation of extracellular matrix, mostly collagen type I, in response to acute or chronic liver injuries 1. The causes of fibrosis include viral hepatitis, steatohepatitis for alcohol or obesity, autoimmune or metabolic disease, drug exposition, and others 1. The etiology of liver injury defines the pattern of fibrosis progression and the involvement of the different populations of myofibroblasts 2.
Although portal myofibroblasts are common in biliary fibrosis, myofibroblasts originated from activation of hepatic stellate cell (HSC) are also important in this process 3. The activated-HSC is the principal cell involved in collagen production. Its activation results in an intense pro-inflammatory and pro-fibrogenic response, which can be induced by apoptotic bodies, reactive oxygen species (ROS), and bacterial endotoxins 4.
Gut-derived bacterial products, like lipopolyssacharides, reach the liver through portal vein and can mediate several immune responses with participation of inflammatory cytokines 5. Bacterial endotoxins can bind to Toll-like receptor 4 (TLR4) present in hepatic cells, which triggers the release of TNFα, IL-6, IL1β, and other inflammatory cytokines 5. This signaling promotes the production of collagen by activated-HSC, mediated by Tgfβ 6. Because of the importance of gut-liver axis in fibrosis progression, it has been hypothesized that the modulation of intestinal microbiota using probiotics could improve the gut barrier and reduce inflammatory and fibrogenic response in liver disease 7) (8. Probiotics are live micro-organisms that, when consumed in adequate amounts, confer a health benefit on the host 9.
The Lactobacillus rhamnosus GG (LGG) is a commensal Gram-positive bacteria widely used as a probiotic strain because of its beneficial effects on the intestinal barrier and inflammatory profile 10) (11. The therapeutic use of LGG is safe and has been extensively studied in allergies, diarrhea, fatty liver disease and other conditions 12) (13) (14 . Bajaj et al. found a reduction in endotoxemia and an improvement in gut dysbiosis in cirrhotic patients treated with LGG 15 . However, the effect of this strain in hepatic fibrosis was not studied. Therefore, the aim of this study was to evaluate the effect of Lactobacillus rhamnosus GG in hepatic fibrosis in a model of chronic cholestatic liver disease in rats.
METHODS
ETHICS STATEMENT
All procedures were conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication number 85-23, revised 1996) of the National Guidelines on Animal Care. In addition, the experimental protocols were approved by the local Ethics Committee of the Hospital de Clínicas de Porto Alegre (Comissão de Ética no Uso de Animais do Hospital de Clínicas de Porto Alegre - no. 12 0312).
ANIMALS AND STUDY DESIGN
Twenty-nine adult male Wistar rats (two months old, 300 g ± 43 g) were obtained from the Experimental Animal Unit of the Research Center of Hospital de Clínicas de Porto Alegre. The rats were allocated four animals per cage and maintained in a controlled environment (room temperature 20 °C ± 2 °C, standard light/dark cycle of 12 hr-lights on at 07:00 am). Standard food and water were provided ad libitum. Animals were randomly distributed into two surgical groups: bile duct-ligated (n = 17) or sham-operated (n = 12). The bile duct ligation procedure was conducted as previously described 16. Rats were anaesthetized with ketamine (90 mg/kg) and xylazine (12 mg/kg) intraperitoneally. After laparotomy, the bile duct was double ligated with non-absorbent surgical sutures and resected between the two ligatures. The sham-operated rats underwent the same surgical procedure with exception of bile duct ligation and resection. Before returning to home-cages, all animals received a subcutaneous injection of tramadol (5 mg/kg).
Two weeks after surgery, the two experimental groups were randomly subdivided into two groups, which received the probiotic Lactobacillus rhamnosus GG ATCC 53103 (LGG) or phosphate buffered saline (PBS). Six sham-operated (Ctrl-P) and eight bile duct-operated (BDL-P) rats received 2.5 x 107 colony-forming units of LGG (Culturelle(tm), Amerifit, USA) in 1 ml of PBS through gavages 12. The other six sham-operated (Ctrl) and nine bile duct-operated (BDL) rats received gavages containing just 1 ml of PBS.
The treatment was performed daily, always during the same period of the day, for two weeks (from the third to the end of the forth week post-surgery). After that time, treatment was discontinued for five days until the sacrifice (33 days after surgery).
SAMPLE COLLECTION
Rats were anesthetized as previously described, blood samples were withdrawn by cardiac puncture from the left ventricle and centrifuged (five minutes at 5,000 × g), and plasma was stored at -80 °C until analysis of the biochemical markers of liver dysfunction. Subsequently, rats received 0.1 ml of heparin (5,000 U/ml) and were perfused with 50 ml (500 ml/h) of PBS at 4 °C, using an infusion pump with a cannula inserted into the heart left ventricle. Liver samples were taken from the median lobe (approximately 50 mg each) and stored at -80 °C for further investigation of gene expression and oxidative stress. The remaining of the liver tissue was stored in a 10% formaldehyde buffered solution for histological examination.
OXIDATIVE STRESS ANALYSES
To determine the reduced thiol (SH) groups present in liver samples, a protocol according to Soszynski et al. 17 was performed. Briefly, free sulfhydryl groups in the liver homogenates samples (40 µl) reacted with 10 µL of 10 mM DTNB (Sigma-Aldrich D8130) (diluted in ethanol), and the formation of the yellowish 2-nitro-5-thiobenzoate was measured by spectrophotometer, at 412 nm. The concentration of color complex protein was determined using the linear equation obtained with a standard curve of reduced glutathione. Results were presented as nmol of SH/mg of protein.
The analysis of catalase (CAT) activity was based on the sample ability in consuming hydrogen peroxide compared to a standard curve built with purified catalase (Sigma-Aldrich C9322). The reaction was followed at 240 nm and expressed as unit/mg protein 18. The superoxide dismutase (SOD) catalyzes the reaction of two superoxide anions resulting in the formation of hydrogen peroxide, which is less reactive and can be degraded by enzymes such as CAT. The analysis of SOD is based on the ability of the sample to inhibit the superoxide-mediated adrenaline oxidation 19. One unit of SOD activity was defined as the amount of enzyme required to inhibit the reaction of oxidation by 50%, measured by absorbance at 480 nm, and was expressed as unit/mg protein. All analyses were normalized by protein content estimated by Bradford method (Bio-Rad #500-0201) 20.
QUANTITATIVE REAL TIME POLYMERASE CHAIN REACTION (q-PCR)
Total RNA from liver samples (50 mg) were isolated using TRIzol(r) reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. First strand cDNA was synthesized from 1.25 µg of total RNA using High Capacity cDNA reverse Transcription Kit (Invitrogen(tm), Life Technologies(tm), Carlsbad, CA, USA) according to the manufacturer's protocol. Gene expression analysis was performed in duplicate, in 48 well StepOne(tm) system (Applied Biosystems(r), Life Technologies(tm), Carlsbad, CA, USA) using TaqMan(r) Gene Expression Assays (Life Technologies(tm), Carlsbad, CA, USA). The following genes were investigated: tumor necrosis factor alpha (Tnfα: Rn01525859_g1), interleukin 6 (IL-6: Rn00561420_m1), Toll-like receptor 4 (Tlr4: Rn00569848_m1), transforming growth factor beta (Tgfβ: Rn00572010_m1), matrix metalloproteinases 2 and 9 (Mmp2: Rn01538170_m1; Mmp9: Rn00579162_m1), and beta-actin (β-actin: Rn00667869_m1) as endogenous control. Each reaction contained 5 µl of TaqMan(r) Gene Expression PCR Master Mix (Life Technologies(tm), CA, USA), 0.5 µl of the probe for each target gene, 2 µl of diluted cDNA (100 ng) in 10 µl of final reaction mixture. The two-step PCR conditions were two minutes at 50 °C, ten minutes at 95 °C, 40 cycles with ten seconds at 95 °C, and one minute at 60 °C. All samples were analyzed in duplicate and gene expression was quantified using the 2-∆∆Ct (threshold cycle) method using the control group as the reference 21.
HISTOLOGICAL AND IMMUNOHISTOCHEMICAL ASSESSMENT
Livers were fixed in 10% buffered formalin for 48 hours, paraffin embedded, and sectioned (3 µm). Five-micrometer-thick sections were used for both immunohistochemistry and picrosirius red staining. To assess biliary ductular reaction, immunohistochemistry assay was performed to label a cytokeratin 7 (CK7), a marker of biliary epithelium in outlining biliary structures 22. Sections were incubated with mouse anti-CK7 primary antibody (Abcam, Cambridge, UK, Ab9021; dilution 1:100), and immunolabeling was amplified using the avidin-biotin-peroxidase complex, as previously described 23. As secondary antibody, a multispecies reagent was used (EasyPath; Erviegas Ltd, São Paulo, Brazil). Picrosirius red was performed in order to evaluate the fibrosis extent.
Three images were photographed (10-fold magnification) for each liver section using Q Capture Pro Software v.5.1.1.14 (Q Imaging Co. Burnaby, BC, Canada) and were quantified using Adobe(r) Photoshop(r) CS3 software. To quantify the positivity CK7 presence and collagen deposition, the color depicting positivity, red for picrosirius staining and brown for CK7 immunohistochemistry, was selected. Color representing the liver parenchyma (green or blue for picrosirius and CK7 immunohistochemistry, respectively) was set as background. The absolute number of pixels of the color of interest and the background were taken, and total positivity was calculated by dividing the total pixels of each image by the number pixels of the color of interest 24.
SAMPLE SIZE CALCULATION AND STATISTICAL ANALYSES
Sample size calculation was based on collagen quantification in liver using picrosirius staining in a pilot study. A sample of five animals was calculated to detect a reduction of 20% in hepatic content of collagen in BDL-P compared to BDL, considering 80% power and 5% significance (Winpepi 11.44).
Statistical analyses were performed by using Prism 5 (Graph Pad, San Diego, USA). Distributions were first tested for normality using the Shapiro-Wilk test. Multiple comparisons were performed using 1-way ANOVA with Tukey-Kramer post hoc tests. Data are presented as mean ± standard deviation. Results with p < 0.05 were considered as significant.
RESULTS
LGG DO NOT AFFECT CLINICAL AND BIOCHEMICAL PARAMETERS OF CHOLESTATIC DISEASE
At the fourth week after surgery, BDL and BDL-P rats exhibited signs of chronic cholestatic liver disease: hepatic enlarged abdomen, yellowish fur and tail, hepatomegaly, and splenomegaly. Plasma biochemical analyses confirmed these findings (Table I). Rats submitted to the bile duct ligation, BDL or BDL-P groups, exhibited higher plasma levels of total bilirrubin, alkalin phosphatase, and gamma glutamyl transferase, accompanied by a reduction of body weight and albumin levels when compared to control groups.
EFFECT OF LGG ON OXIDATIVE STRESS PARAMETERS
The hepatic oxidative stress was evaluated by determining activities of SOD and CAT and by measuring of SH groups (Fig. 1). Bile duct ligation induced a significant reduction in CAT activity when compared to the Ctrl (p < 0.001) (Fig. 1A) and Ctrl-P (p < 0.001) (Fig. 1A) but no difference was detected between BDL and BDL-P. In contrast, no changes were found for SOD activity among groups (p = 0.1025) (Fig. 1B). Increased SOD/CAT ratio can be related to a pro-oxidative status because hydrogen peroxide, the product of SOD reaction, is not fully transformed in H2O and O2. The BDL group had a higher SOD/CAT ratio when compared to Ctrl (p < 0.05) (Fig. 1C) and Ctrl-P (p < 0.01) (Fig. 1C). The treatment with LGG promoted a reduction of about 30% in SOD/CAT ratio in comparison to BDL group, albeit not significant (p = 0.398) (Fig. 1C). SH levels were higher in the BDL group compared to Ctrl (p < 0.05) (Fig. 1D) and Ctrl-P (p < 0.01) (Fig. 1D), without any difference between BDL-P in comparison to both control groups.
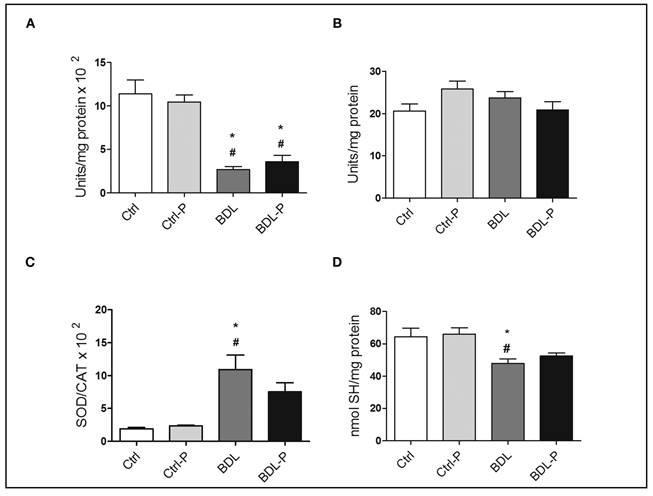
Figure 1 The effect of LGG on oxidative stress parameters. The antioxidant defenses were measured by determining activities of SOD (A) and CAT (B). The SOD/CAT ratio (C) was used to evaluate the pro-oxidative status and the measuring of SH groups (D) to measure the oxidative damage. To test differences between groups, ANOVA followed by the Tukey test was used (p < 0.05). *Difference versus Ctrl. #Difference versus Ctrl-P.
LGG ALTERS INFLAMMATORY PATHWAYS IN HEPATIC TISSUE
The mRNA expression of liver inflammatory and fibrosis markers was not altered in Ctrl animals treated with LGG (Fig. 2). However, BDL model consistently presented increased levels of Tlr4 (1.5-fold, p < 0.05) (Fig. 2A), Tnfα (3.9-fold, p < 0.001) (Fig. 2B), IL-6 (12.8-fold, p < 0.01) (Fig. 2C), Tgfβ (1.8-fold, p < 0.01) (Fig. 2D), Mmp2 (3.2-fold, p < 0.05) (Fig. 2E), and Mmp9 (3.5-fold, p < 0.05) (Fig. 2F). When the BDL group was treated with LGG (BDL-P group), no reduction could be seen for all markers but IL-6, which was 30% less expressed in BDL-P than in BDL group (p < 0.05) (Fig. 2C).
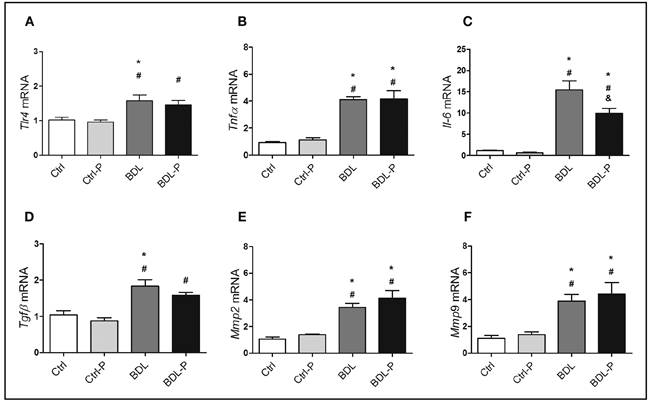
Figure 2 The effect of LGG treatment on genes involved in inflammatory and fibrogenic pathways in hepatic tissue. The gene expression of Tlr4 (A), Tnfα (B), Il-6 (C), Tgfβ (D), Mmp2 (E), and Mmp9 (F) were determined by q-PCR and are expressed 2-∆∆Ct. Differences were tested by ANOVA followed by the Tukey (p < 0.05). *Difference versus Ctrl. #Difference versus Ctrl-P. &Difference versus BDL.
LGG REDUCES HEPATIC COLLAGEN DEPOSIT AND DUCTULAR REACTION
As expected, the content of collagen increased 12-fold in the BDL group in comparison to the Ctrl group (p < 0.01) (Fig. 3). The treatment with LGG significantly promoted a reduction of 32% in hepatic collagen deposition in comparison with BDL group (p < 0.05) (Fig. 3). Concerning the ductular reaction, the BDL group had a higher (29-fold) immunocontent of CK7 when compared to Ctrl (p < 0.001) (Fig. 4). LGG treatment reduced CK7 in 39% in relation to the BDL group (p = 0.036) (Fig. 4).
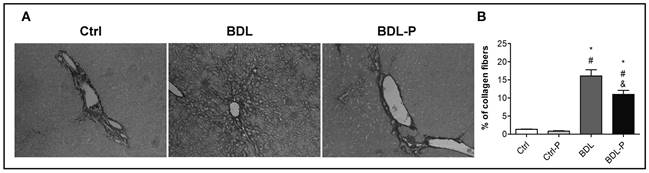
Figure 3 The effect of LGG on collagen deposition. Liver sections were stained with Picrosirius Red (original magnification 400×). A. Representative photomicrographs of liver sections of Ctrl, BDL, and BDL-P group, respectively. The quantification of stained collagen is shown in B. To test differences between groups, ANOVA followed by the Tukey test was used (p < 0.05). *Difference versus Ctrl. #Difference versus Ctrl-P. &Difference versus BDL.

Figure 4 The effect of LGG on ductular reaction. The ductular reaction was evaluated by CK7 immunohistochemistry. All images were captured at 400× final magnification. A. Representative photomicrographs of liver sections of Ctrl, BDL, and BDL-P group, respectively. The quantification of immunocontent of CK7 is shown in B. To test differences between groups, ANOVA followed by the Tukey test was used (p < 0.05). *Difference versus Ctrl. #Difference versus Ctrl-P. &Difference versus BDL.
DISCUSSION
In this study, the effects of the LGG treatment on the fibrosis progression and ductular reaction in a model of cholestatic disease are shown. Our data support the hypothesis that probiotic treatment can reduce collagen deposition, CK7 content, and IL-6 expression in the liver.
Here, LGG treatment started after 14 days of bile duct ligation to investigate the effect of probiotic on an established cholestatic liver disease. As previously showed by Georgiev et al., liver changes caused by BDL model occurred within the firsts 14 days, with no further significant collagen accumulation after that time, demonstrating that this time period is suitable for the study of cholestatic chronic disease 25. As expected, a significant increase in classical markers of cholestasis in rats subjected to bile duct ligation were found 25. Nonetheless, the treatment with probiotic did not lead to changes in the overall clinical manifestation or in the biochemical parameters of biliary flow. Zhou et al. found decreased levels of total bilirrubin evaluated in portal serum of bile duct-ligated rats (for ten days) after treatment with 2 × 108 CFU/ml of L. plantarum 26. However, we evaluated plasma parameters in blood collected by cardiac puncture in rats with advanced liver disease (33 days after ligation). The difference found in the blood collection site and the stage of the disease could explain why we did not find similar results to Zhou et al.
According to Lee et al., damages in hepatocyte or biliary epithelium is a sine qua non condition to the development of a chronic hepatic disease that is followed by an intense inflammatory and fibrogenic response 1. As portal fibroblasts are located adjacent to bile duct epithelia, they are mostly implicated in biliary cirrhosis and known as the first responders in the obstructive liver disease 3) (27. Portal fibroblasts produce and secrete Tgfβand inflammatory cytokines that can induce the transdifferentiation of HSC into myofibroblasts, which are responsible for a later cellular response in cholestatic disease 3) (27) (28. Myofibroblasts can directly contribute to the persisting inflammation by releasing pro-inflammatory mediators or acting as a target of ROS, cytokines or endotoxins 2. In our study, animals subjected to a BDL procedure have increased oxidative stress, as seen by the reduction of CAT activity and SH content. SOD and CAT act coordinately to control ROS levels and the SOD/CAT activity ratio gives an idea of enzymatic antioxidant equilibrium 29. The BDL group had a SOD/CAT ratio five times higher than control groups, showing a potentially oxidative environment, as confirmed by the decrease in SH levels. LGG treatment presented slight improvement in SOD/CAT ratio, although not statistically significant, which can be related to the decrease of activated inflammatory cells.
Intestinal dysbiosis and bacterial overgrowth are common in chronic liver disease and can contribute to inflammatory response by activation of the Tlr family, especially by lipopolysaccharides 1. Indeed, we found an increase in Tlr4, Tnfα, and II-6 gene expression in liver of BDL rats, as previously reported 30) (31) (32. Some studies have demonstrated that probiotic treatment decreases Tlr4, Tnfα, and II-6 expression in animal model of CCl4 induced-fibrosis 30) (31. In the same way, a reduction in plasma TNFα and IL-1α levels in a BDL model with administration of probiotic VSL#3 (50 billion bacteria/kg of body weight/day) was demonstrated 32. It is important to note that these studies started probiotic treatment at the onset of the disease. On the contrary, we used probiotics to treat an established liver disease, which could lead to more slight results. Furthermore, the treatment with probiotics was discontinued for five days before the sacrifice in our study. We chose this design in an attempt to find out which cytokines would be expressed even after the end of the treatment, however, this may have hidden some pathways activated by LGG use. These strategies used in our experimental design may have affected the magnitude of the results since it was not possible to show a significant decrease in inflammatory markers, except for II-6 gene expression.
Bacterial compounds derived from the gut can activate Kupffer cells to produce IL-6 in the periportal region, leading to HSC activation and liver fibrosis 33) (34) (35. We suggest that LGG treatment can reduce the gut delivered endotoxins, decreasing an exposure of Kupffer cells and, consequently, reducing II-6 expression. Nonetheless, IL-6 has a controversial role in fibrosis. Although serum and hepatic levels of IL-6 are higher in patients and animals with acute or chronic liver disease, this cytokine has been associated with protective functions during hepatic fibrogenesis 36. This idea is supported by studies where II-6-deficient animals presents an uncommon higher liver fibrosis, which is restored after IL-6 administration 33. However, in humans with biliary fibrosis, IL-6 mediates the ductular reaction and is released specifically by biliary epithelia and portal fibroblasts in contrast to other forms of fibrosis 3. In addition, in vitro experiments found that IL-6 derived from Kupffer cells promotes survival and proliferation of HSC, followed by enhancement of liver fibrosis 37) (38. Wang et al. 37 suggested that IL-6 can positively or negatively regulate liver fibrosis via targeting different types of liver cells, since IL-6 receptors are broadly expressed in hepatic cells. Despite that, in the present study reduction of II-6 was observed in the BDL-P group, which was accompanied by a decrease in collagen deposition and ductular reaction.
Myofibroblasts are the major cells responsible for excess deposition of extracellular matrix in response to Tgfβreleased by Kupffer cells and HSC 2. Also, cells involved in the ductular reaction in human chronic biliary diseases can express Tgfβ 39. We showed that BDL groups had an increase of about 75% in Tgfβ expression, which was accompanied by a significant increase in hepatic fibrosis (12-fold) and ductular reaction (29-fold) when compared with control. The treatment with LGG in BDL animals promoted a significant reduction in the collagen deposition and ductular reaction. In the same way, Tgfβ was 25% less expressed in BDL-P in comparison with the BDL group, albeit not statistically significant.
Some limitations of this study should be addressed. First, this study is descriptive in nature and causal relations should be drawn with care. However, for the first time, we showed that LGG treatment could be used to attenuate ductular reaction and fibrosis in a model of established liver disease. Second, we suggest that LGG may impair gram-negative bacteria overgrowth, which in turn would increase HSC activation and fibrosis. Unfortunately, endotoxin measurement could not be presented in this study.
In the present study we demonstrated that LGG administration was effective in attenuating fibrosis in a model of established cholestatic liver disease in rats. The reduction in pro-inflammatory cytokine II-6 might be related to the mechanisms by which probiotics exert their beneficial effects. However, further investigation is necessary to better understand these pathways. Based on our results, we suggest that Lactobacillus rhamnosus GG can be a promising therapy for adjuvant treatment of hepatic fibrosis.















