Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.34 no.6 Madrid nov./dic. 2017
https://dx.doi.org/10.20960/nh.1004
Dietary intake and zinc status in amyotrophic lateral sclerosis patients
Ingesta dietética y estado de zinc en pacientes con esclerosis lateral amiotrófica
Heloisa Fernanda Lopes da Silva1, Acsa Nara de Araújo Brito1, Erika Paula Silva de Freitas1, Mário Emílio Teixeira Dourado Júnior2, Karine Cavalcanti Maurício de Sena-Evangelista1 and Lúcia Leite-Lais1
Departments of 1Nutrition and 2Medicine. Federal University of Rio Grande do Norte. Natal-RN, Brazil
ABSTRACT
Background: There is considerable evidence that abnormal zinc homeostasis is related to amyotrophic lateral sclerosis (ALS) pathogenesis, and malnutrition is an independent prognostic factor for worsened survival of ALS patients.
Objective: To evaluate the dietary intake and zinc status in patients with ALS, treated in a specialized outpatient facility in Natal, Brazil.
Methods: Twenty patients with ALS (case group) and 37 healthy subjects (control group) were included. Clinical and anthropometric assessments were carried out and dietary intake was obtained from two 24-hour recalls. Plasma and urinary zinc concentrations were determined by atomic absorption spectrophotometry.
Results: Most of the participants were eutrophic. Mean energy, protein, carbohydrate and fat intake was significantly lower for the case group. There was greater prevalence of inadequate zinc intake in the case group (35%) compared to controls (27%). Mean plasma zinc was significantly lower in the case group than in controls (77.13 ± 22.21 vs 87.84 ± 17.44 µgZn/dl). Urinary zinc did not differ significantly between cases and controls. In the case group, plasma and urinary zinc concentrations were below reference values in 50.0% and 52.6% of patients, respectively.
Conclusion: A large portion of patients with ALS exhibited poor dietary intake and changes in body zinc status. The zinc deficiency found in half of the ALS patients may contribute to a worsened prognosis and should be the target of nutritional intervention that aims to correct this deficiency.
Key words: Amyotrophic lateral sclerosis. Dietary intake. Zinc. Nutritional status.
RESUMEN
Introducción: hay pruebas considerables de que los cambios en la homeostasis del zinc están relacionados con la patogénesis de la esclerosis lateral amiotrófica (ELA) y que la malnutrición es un factor pronóstico capaz de reducir la supervivencia de los pacientes con ELA.
Objetivo: evaluar la ingesta dietética y el estado de zinc en pacientes con ELA, tratados en un centro de atención ambulatoria especializado en Natal, Brasil.
Métodos: se incluyeron 20 pacientes con ELA (grupo de casos) y 37 sujetos sanos (grupo control). Se realizaron evaluaciones clínicas y antropométricas y se obtuvo la ingesta dietética en dos recordatorios de 24 horas. Las concentraciones plasmáticas y urinarias de zinc se determinaron por espectrofotometría de absorción atómica.
Resultados: la mayoría de los participantes fueron eutróficos. El consumo medio de energía, proteínas, carbohidratos y grasas fue significativamente menor en el grupo de casos. Hubo una mayor prevalencia de ingesta inadecuada de zinc en el grupo de casos (35%) en comparación con los controles (27%). El zinc plasmático medio fue significativamente menor en el grupo de casos que en los controles (77,13 ± 22,21 frente a 87,84 ± 17,44 μgZn/dl). El zinc urinario no difirió significativamente entre los casos y los controles. En el grupo de casos, las concentraciones de zinc plasmático y urinario fueron inferiores a los valores de referencia en el 50,0% y 52,6% de los pacientes, respectivamente.
Conclusión: gran parte de los pacientes con ELA exhibieron una ingesta dietética pobre y modificación en el estatus de zinc corporal. La deficiencia de zinc encontrada en la mitad de los pacientes con ELA puede contribuir a un empeoramiento del pronóstico y debe ser el objetivo de la intervención nutricional que apunta a corregir esta deficiencia.
Palabras clave: Esclerosis lateral amiotrófica. Ingesta dietética. Zinc. Estado nutricional.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neuromuscular disease characterized by loss of upper and lower neurons in the cerebral cortex, brain stem and spinal cord, leading to muscle atrophy, paralysis and death (1). Around 90-95% of ALS cases are sporadic, with no known cause. The remaining 5-10% result from hereditary genetic mutations and are known as familial ALS. Around 20% of familial ALS cases are associated with mutations in the gene that codifies the superoxide dismutase 1 (SOD1) antioxidant enzyme (2).
The pathogenesis of ALS is complex and not entirely known. It is believed that the combination of different mechanisms may be involved in motor neuron injury, including oxidative stress, glutamate excitotoxicity, mitochondrial dysfunction, neuroinflammation, apoptosis, protein aggregation and genetic mutations (3,4). In addition, zinc plays a key role in all these mechanisms associated with ALS pathogenesis (3). Besides its antioxidant, anti-inflammatory and immunomodulatory properties, zinc is essential for the central nervous system, since it is involved in neurogenesis, synaptogenesis, neuronal growth and neurotransmission (5).
The majority of ALS patients do not achieve a satisfactory dietary intake, due to the presence of factors such a dysphagia, inappetence, depression and socioeconomic limitations. This chronic negative balance between nutrient intake and requirements leads to a high prevalence of malnutrition and worse prognosis (6). Thus, considering the importance of zinc in ALS and the scarcity of studies on this issue, the aim of the present study was to evaluate the dietary intake and zinc status in patients with ALS treated in a specialized outpatient facility at the Hospital Universitário Onofre Lopes (HUOL) in Natal, Brazil.
MATERIALS AND METHODS
Study design and selection criteria
This is an unmatched case-control study that was approved by the Research Ethics Committee of the Hospital Universitário Onofre Lopes (CAAE 40467214.0.0000.5292). Informed consent was obtained from all individual participants included in the study. The case group consisted of patients of both sexes, diagnosed with ALS and treated at the ALS/HUOL outpatient facility between March 2015 and February 2016. Individuals without definitive diagnosis, those using micronutrient supplements, with inflammatory intestinal diseases, kidney failure, liver failure, biliary diseases, diabetes, or other associated neurological diseases were excluded due to the possible interference of zinc in the biochemical profile. The control group, selected at the HUOL nutrition outpatient facility, was composed of healthy people of both sexes aged 20 years or older, taking no medication or micronutrient supplements.
Clinical and nutritional data
Case group patients were clinically characterized in terms of symptom onset (bulbar or spinal), symptom duration (in months), feeding pathway (oral and/or enteral) and score on the ALS functional rating scale (ALSFRS) (7). This instrument assesses the motor, bulbar and respiratory function of these patients, serving as a prognostic indicator. Scores range from 0 (worst function) to 48 (best function) points. For both groups, body mass index (BMI) was determined. The BMI of patients was classified according to cut-off scores recommended by the World Health Organization (WHO) (8).
Dietary intake assessment
Habitual dietary intake was investigated using two non-consecutive 24-hour recalls for each patient, obtained from weekdays, 30 to 45 days apart (9). Dietary energy, protein, fat, carbohydrate, fiber and zinc intake were measured applying Virtual Nutri Plus® 2.0 software. Food items or preparations that were not found in the software's databank were added based on data contained in Brazilian (10,11) and international (12) food composition tables and/or the nutrition labels of industrialized products, including the enteral formulas. Habitual food intake of the group was estimated using statistical tests to obtain energy and nutrient values adjusted for intra and interpersonal variability, according to Slater et al. (13). Next, nutrients were adjusted for energy, applying the residual method described by Willet and Stampfer (14).
To determine the dietary intake adequacy of ALS patients, the following nutritional recommendations were considered (15): energy, 35 kcal/kg/day; protein, 1.5 g/kg/day; fat, 30% of total calories; carbohydrates, sufficient to complete the total calories; and fibers, 20-30 g/day. The estimated average requirement (EAR) was used to assess zinc intake (9.4 mg/day for men and 6.8 mg/day for women) (16). Analysis of dietary zinc was presented as prevalence of inadequacy, obtained by the EAR method as cut-off point (17).
Assessment of plasma and urinary zinc
After an overnight fast, 5-10 ml blood samples were collected from participants in a tube with 30% sodium citrate anticoagulant (100 µl). The samples were centrifuged at 3,000 rpm for 15 minutes, at 4 ºC, and the plasma was separated and stored in a freezer at -20 ºC until analysis. The 24-hour urine samples were collected by the subjects in a supplied container and kept refrigerated until delivery. These were homogenized and a 25 mL sample was stored in a freezer at -20 ºC until analysis. All procedures related to manipulation of zinc samples were performed according to international standards for prevention of zinc contamination of the environment (18).
Plasma and urinary zinc concentrations were determined by atomic absorption spectrophotometry (SpectrAA200, Varian, Victoria, Australia), considering a wavelength of 213.9 nm, slit 1.0 nm, amperage 5.0 mA, expansion factor 1.0 and sample flow of 5 mL/min. The calibration curves were prepared with Titrisol® standard solution (Merck, Germany) at the following concentrations: 0.00, 0.10, 0.20, 0.30, 0.50 and 1.00 µg/ml, and then refrigerated. The solution was diluted in Milli-Q® water and 10% glycerol was used only for the plasma curve in order to correct the difference in the matrix between the standard and the sample. Analyses were conducted in duplicate and the results were calculated from the average of the readings of the concentrations obtained, establishing a coefficient of variation of less than 10%. Seronorm™ Trace Elements Serum L-1 standard (Sero AS, Billingstad, Norway) was used as a reference for zinc analysis.
The analytical determination of plasma zinc was carried out in line with the method proposed by Rodrigues et al. (19). The results were expressed in µgZn/dl. A range of 70-110 µg/dl was considered as reference value for plasma zinc (20). Analytical determination of urinary zinc followed the method proposed by Kiilerich et al. (21). Results were expressed in µg/24h, based on the average concentration obtained multiplied by the total volume of 24-hour urine. A range of 300-600 µg/24h was considered as reference value for zinc excretion in urine (20).
Statistical analysis
The data obtained were analyzed using Stata 14.0 software. The distribution of continuous variables was visually assessed by constructing histograms. The data were presented as mean ± standard deviation (SD), mean (95% confidence interval [CI]) and median (interquartile range), when appropriate. Countings and proportions were used to summarize categorical variables. The Student's t-test for independent samples and Fisher's exact test were applied to test differences between continuous variables and proportions, respectively. The difference was adjusted for covariables using simple and multiple linear regression models. Seven multiple linear regression models, including the covariables age, sex, BMI and dietary zinc intake, were created to eliminate possible confounding factors in terms of the analysis of plasma and urinary zinc parameters. One and two data items, missing for urinary zinc and BMI, respectively, were submitted to multiple imputation. Pearson's coefficient was computed using a simple linear regression coefficient for variables whose observations were inputted. In these analyses, the variables were centralized in their means and standardized by the standard deviation of the sample. Percentiles 25, 50 and 75 for variables with imputed data were constructed based on quantile regression models without a predictor variable. A significance level of 5% was adopted for all analyses.
RESULTS
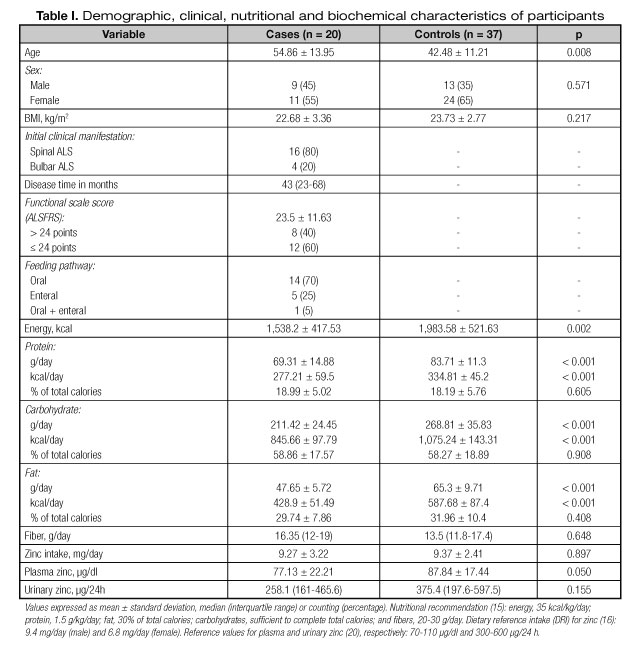
The demographic, clinical, nutritional and biochemical information of participants are shown in table I. The average age of participants was significantly lower in the control group compared to the case group. There was a predominance of women in both case and control groups. Mean BMI was similar between the groups, with 78.9% of cases and 62.2% of controls at normal range (eutrophic). According to the ALS Functional Rating Scale (ALSFRS), 65% of ALS patients showed some level of dysphagia.
The average dietary intake adjusted for energy and macronutrients was significantly lower for the case group compared to controls. By contrast, the average zinc and fiber intake did not differ significantly between the groups (Table I). Although there was no significant intergroup difference in zinc intake, a higher inadequate prevalence was found in the case group (35%) as compared to the control group (27%). Individual analyses of the case group also showed that most patients with ALS exhibited poor dietary intake of energy, protein, lipid, carbohydrate and fiber (Fig. 1).
Mean plasma zinc was significantly lower in the case group as compared to the control group (Table I). There was a tendency to lower urinary zinc in the case group; however, no statistical difference was observed (Table I). Individual analysis showed that more cases obtained below reference plasma and urinary zinc values, represented by 50.0% and 52.6% of cases vs 13.5% and 37.8% of controls, respectively.
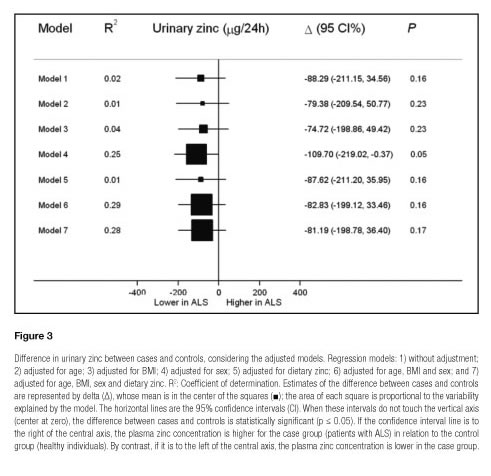
The regression models with several adjustment levels for plasma and urinary zinc are illustrated in figures 2 and 3, respectively. After adjusting for the main covariables, the plasma zinc values differed marginally between cases and controls, except for model 2, suggesting the influence of age on plasma zinc. Moreover, the models explained only 4 to 9% of variability in zinc plasma (Fig. 2). The graphic analysis of the regression models for urinary zinc excretion shows no difference between cases and controls, except for model 4, which was marginally significant, confirming the influence of sex on urinary zinc excretion. The high variability of urinary zinc can be observed in models 4 (25%), 6 (29%) and 7 (28%). Furthermore, age and BMI were independent predictors of urinary zinc excretion (Fig. 3).
DISCUSSION
ALS generally affects individuals between the ages of 50 and 60 years (2). The average age of cases studied was in this range; however, 35% were younger than 50 years. According to the scientific literature, men are slightly more affected than women, with a male to female ratio about 1.6:1 (1). In the present study, albeit not statistically significant, most cases were women, similar to the study by Nicoletti et al. (22), which also found a higher prevalence of spinal ALS as initial manifestation.
In our study, most patients with ALS were eutrophic (mean BMI = 22.68 kg/m2) (Table I) despite low food intake, indicating possible overweight or obesity before diagnosis (data not investigated). Moreover, the multidisciplinary care may have contributed for a better nutritional status of our patients as shown by Rooney et al. (23). The maintenance of a eutrophic BMI is of the utmost importance, since there is a U-shaped association between BMI and mortality in ALS patients (24).
In the present study, 84.2% of the cases had energy intake below the recommended levels (Fig. 1).
Similarly, Genton et al. (6) found that energy intake was below the recommended levels in 70% of ALS patients. Patients with ALS progressively develop muscle weakness, muscle atrophy and dysphagia, which makes them vulnerable to insufficient energy intake (25,26). Negative energy balance in ALS contributes to degeneration of motor neurons (27) and micronutrient deficiency (28). The high rate of difficulty in swallowing and the low rate of enteral feeding found in our study may have contributed to the patients' poor dietary intake. Thereby, gastrostomy may be used to correct insufficient oral intake and has been associated with maintaining weight and improving survival (26).
Although mean zinc intake did not differ between groups, greater prevalence of inadequate zinc intake was observed for the case group, which also showed lower energy and protein intake. This unsatisfactory dietary intake both in nutritional quantity and quality contributes to weight loss, malnutrition, and poor prognosis (29). It is estimated that malnutrition in patients with ALS increases the relative risk of death 7.7 fold (30) and that for every 5% weight loss, the risk of death rises by 30% (31).
Furthermore, low zinc intake may influence the pathogenic mechanisms in ALS (3).
For adequate body zinc status, a healthy eating pattern with a regular intake of foods with high bioavailable zinc (oysters, meat, liver, milk, eggs, etc.) is recommended. Enteral formulas are well balanced for micronutrient intake and most of them achieve zinc requirements in 1,500 kcal (32).
Fifty percent of the cases exhibited plasma zinc concentrations below reference values, suggesting zinc deficiency. Despite fluctuations in zinc intake, its concentration in plasma is strongly regulated. Thus, only prolonged low zinc intake or chronic poor zinc absorption is capable of reducing plasma values, suggesting deficiency (33). Moreover, due to the strong homeostatic control of zinc, the deficiency detectable at a plasma level is considered as severe, since normal serum zinc values can be found in marginal zinc deficiency (34).
Models 4, 6 and 7 (Fig. 3) explain a high proportion of zinc variability in urine. The marginal significance demonstrated only in model 4 (p = 0.049) confirms the influence of sex on urinary zinc excretion, given that differences in body composition between men and women show greater zinc excretion in men (35). This reinforces the importance of zinc excretion correction by this covariable. The low levels of urinary zinc found in most of the controls (52.6%) show a possible attempt to retain more zinc and compensate for the reduced zinc concentration in plasma (20).
Szewczyk (36) reports that zinc deficiency increases the risk of neurodegenerative diseases and is prevalent in neurological patients. Corroborating this, Roos et al. (37) found low plasma zinc content in patients with ALS. Moreover, Peters et al. (38), in a case-control study, observed an inverse association between serum zinc concentration and ALS. This association was stronger in those with worse function, suggesting that zinc may play a role in the etiology of ALS and that supplementation with this mineral may benefit these patients.
Our results suggest that zinc deficiency is a condition inherent to ALS, independently of covariables. The poor dietary intake and the zinc deficiency detected in patients with ALS may contribute to a worse prognosis and should be the target of specific nutritional intervention aimed at correcting the deficiency. There are limitations in the present study. Because of the scarcity of the disease, the number of case group patients was low. This may have compromised the statistical power of the analysis and its representativeness for other populations.
CONCLUSION
Compared to the control group, patients with ALS showed lower energy and macronutrient intake, higher prevalence of inadequate zinc intake, lower plasma zinc concentration, as well as tendency to lower urinary zinc excretion.
ACKNOWLEDGEMENTS
This work was supported in part by the Foundation for Research Support of Rio Grande do Norte (FAPERN), Brazil.
REFERENCES
1. Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet 2009;369:2031-41.
2. Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol 2009;65(Suppl 1):S3-S9.
3. Smith AP, Lee NM. Role of zinc in ALS. Amyotroph Lateral Scler 2007;8(3): 131-43.
4. Lasiene J, Yamanaka K. Glial cells in amyotrophic lateral sclerosis. Neurol Res Int 2011:718987.
5. Tyszka-Czochara M, Grzywacz A, Gdula-Argasi?ska J, Librowski T, Wili?ski B, Opoka W. The role of zinc in the pathogenesis and treatment of central nervous system (CNS) diseases. Implications of zinc homeostasis for proper CNS function. Acta Pol Pharm 2014;71(3):369-77.
6. Genton L, Viatte V, Janssens JP, Héritier AC, Pichard C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clin Nutr 2011;30(5):553-9.
7. Guedes K, Pereira C, Paiva K, Valério BCO. Cross-cultural adaptation and validation of ALS Functional Rating Scale-Revised in Portuguese language. Arq Neuro-Psiquiatr 2010;68(1):44-7.
8. World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000.
9. Thompson FE, Byers T. Dietary assessment resource manual. J Nutr 1994;124:2245S-317S.
10. Núcleo de Estudos e Pesquisas em Alimentação (NEPA); Universidade Estadual de Campinas (UNICAMP). Tabela Brasileira de composição de alimentos - TACO. São Paulo: NEPA/UNICAMP; 2011.
11. Philippi ST. Tabela de Composição de Alimentos: suporte para decisão nutricional. Brasília: ANVISA, FINATEC/NUT; 2001.
12. U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25, 2012.
13. Slater B, Marchiony Dl, Fisberg RM. Estimando a prevalência de ingestão inadequada de nutrientes. Rev Saúde Publ 2004;38(4):599-605.
14. Willett WC, Stampfer MJ. Total energy intake: Implications for epidemiological analyses. Am J Epidemiol 1986;124:17-27.
15. Brito ANA, Vale SHL, Alves CX, Castro JL, Dourado Júnior MET, Leite LD. Protocolo diferenciado para Terapia Nutricional na Esclerose Lateral Amiotrófica. Rev Bras Ciênc Saúde 2014;18(1):79-86.
16. Food and Nutrition Board. Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington: National Academy of Sciences; 2001.
17. Beaton GH. Approaches to analysis of dietary data: Relationship between planned analyses and choice of methodology. Am J Clin Nutr 1994;59(Suppl):253-61.
18. King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, et al. Biomarkers of Nutrition for Development (BOND) - Zinc review. J Nutr 2016;146(4):858S-85S.
19. Rodríguez MP, Narizano A, Demczylo V, Cid A. A simpler method for the determination of zinc human plasma levels by flame atomic absorption spectrophotometry. At Spectrosc 1989;10(2):68-70.
20. Gibson RS. Assessment of chromium, copper and zinc status. In: Gibson RS Principles of nutritional assessment, 2nd ed. New York: Oxford University Press; 2005. pp. 683-748.
21. Kiilerich S, Christensen MS, Naestoft J, Christiansen C. Determination of zinc in serum and urine by atomic absorption spectrophotometry; relationship between serum levels of zinc and proteins in 104 normal subjects. Clin Chim Acta 1980;105:231-9.
22. Nicoletti A, Vasta R, Venti V, Mostile G, LoFermo S, Patti F, et al. The epidemiology of amyotrophic lateral sclerosis in the Mount Etna region: A possible pathogenic role of volcanogenic metals. Eur J Neurol 2016;23(5):964-72.
23. Rooney J, Byrne S, Heverin M, Tobin K, Dick A, Donaghy C, et al. A multidisciplinary clinic approach improves survival in ALS: A comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry 2015;86(5):496-501.
24. Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011;44(1):20-4.
25. Ngo ST, Steyn FJ, McCombe PA. Body mass index and dietary intervention: Implications for prognosis of amyotrophic lateral sclerosis. J Neurol Sci 2014;340(1-2):5-12.
26. Kasarskis EJ, Mendiondo MS, Matthews DE, Mitsumoto H, Tandan R, Simmons Z, et al. ALS Nutrition/NIPPV Study Group. Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. Am J Clin Nutr 2014;99(4):792-803.
27. Mattson MP, Cutler RG, Camandola S. Energy intake and amyotrophic lateral sclerosis. Neuromolecular Med 2007;9(1):17-20.
28. Leite LD, Castro JL, Dourado Jr. MET, Brandão-Neto J. Food intake as a parameter of nutritional assessment in amyotrophic lateral sclerosis patients. Rev Bras Nutr Clin 2012;27(2):87-92.
29. Dupuis L, Pradat PF, Ludolph AC, Loeffler JF. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol 2011;10(1):75-82.
30. Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology 1999;53(5):1059-63.
31. Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 2011;82(6):628-34.
32. Iacone R, Scanzano C, Santarpia L, D'Isanto A, Contaldo F, Pasanisi F. Micronutrient content in enteral nutrition formulas: Comparison with the dietary reference values for healthy populations. Nutr J 2016;15:30.
33. King JC. Zinc: An essential but elusive nutrient. Am J Clin Nutr 2011; 94(2): 679S-84S.
34. Wood RJ. Assessment of marginal zinc status in humans. J Nutr 2000;130(5S Suppl):1350S-4S.
35. Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25(1Suppl 2):S99-203.
36. Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci 2013;5:33.
37. Roos PM, Vesterberg O, Syversen T, Flaten TP, Nordberg M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol Trace Elem Res 2013;151(2):159-70.
38. Peters TL, Beard JD, Umbach DM, Allen K, Keller J, Mariosa D, et al. Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology 2016;54:119-26.
 Correspondence:
Correspondence:
Lúcia Leite Lais.
Department of Nutrition.
Federal University of Rio Grande do Norte.
Campus Universitario. Lagoa Nova.
59078-900 Natal-RN, Brazil
e-mail: ludl10@hotmail.com
Received: 30/01/2017
Accepted: 11/04/2017