INTRODUCTION
Obesity is a chronic disease that affects the population throughout the world, being more prevalent than malnutrition and infectious diseases 1. Studies reveal that obesity affects not only adults but is also verified in children and adolescents 2) (3, when related metabolic alterations may have its onset 4. Adipose tissue is characterized, in particular, by its complexity. It would originally be related to energy source and thermogenesis, depending on its differentiation in white adipose tissue (WAT) or brown adipose tissue (BAT), respectively. However, other functions have been attributed to adipose tissue, resulting from its active involvement in immune, hormonal and metabolic processes through the synthesis and release of substances called adipokines 5. Adipose tissue is recognized as an endocrine organ for its secretory and synthesizing function of leptin, estrogens, angiotensinogen and adiponectin, by the ability to establish connections with the central nervous system, in addition to the release of acute-phase proteins and pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF-1) 6. TNF-α and IL-6 cytokines are related to obesity and reduced insulin sensitivity 7. Insulin resistance would lead to increased synthesis and storage of triglycerides in hepatocytes, and thus play an important role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD), a disease with a high potential for cirrhosis and/or hepatocellular carcinoma 8. As a consequence, this population could develop other metabolic alterations such as dyslipidemias, hypertension and type 2 diabetes mellitus (T2DM), secondary to a supposed inflammatory state intrinsic to obesity that would respond by the expression of these comorbidities, according to studies described in adults 9. Considering these aspects, the purpose of this study was to describe the behavior of proinflammatory cytokines in obese children and adolescents with and without non-alcoholic fatty liver disease (NAFLD).
METHODS
A cross-sectional study involving 90 obese children and adolescents of both sexes, aged between eight and 18 years, consecutively evaluated at the Pediatric Nutrology Service of the Federal University of Bahia from January to December 2015, was carried out, featuring a convenience sample. Patients with conditions predisposing to overweight such as Cushing's syndrome, growth hormone deficiency, hypothyroidism, and syndromic obesity were excluded. There was no report of consumption of alcoholic beverages among participants. The body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m) (kg/m2). The z-score of the BMI indicator/age and gender was used to classify the participants' anthropometric status as obese (BMI/i > +2) and severe obese (BMI/i > +3) 10. Waist circumference was obtained with a soft and inelastic tape, at the midpoint between the last rib and the anterior iliac crest 11. GE LOGIC P6 ultrasound with a convex transducer of 2 to 5 MHz was used for the diagnosis and classification of steatosis in grades I, II and III, according to the alteration of echogenicity, and the identification of intrahepatic vessels and diaphragm, according to Hamaguchi et al. 12. The imaging examinations were performed at the research institution and by the same professional. This study was approved by the Ethics Committee from the Federal University of Bahia, Brazil. A children verbal assent and a written consent for participation were obtained through their legal representatives.
LABORATORY ANALYSES
A fasting venous blood sample was taken of all participants for laboratory determinations. The enzymes alanine transaminase (ALT), aspartate transaminase (AST) and gamma-glutamyl transpeptidase (gamma-GT) were measured by the calorimetric kinetic method and expressed in U/l. Altered fasting plasma glucose ≥ 100 mg/dl was assessed by the hexokinase method (Wiener lab.) 13. Insulin levels were evaluated by enzyme linked immunoenzymatic assay (ELISA). Any values greater than 15.0 μUI/ml were considered as abnormal 14. The homeostasis model assessment-insulin resistance (HOMA-IR) index = fasting glucose (mg/dl) x fasting insulin (µIU ml-1)/405 was used to identify insulin resistance 15. We used the cut-offs adjusted by gender and age according to Almeida et al., summing the mean value to two standard deviations 16. The high sensitivity C-reactive protein (hsCRP) was determined by immunoturbidimetric method. The interleukin-2 (IL-2), interleukin-6 (IL-6), interferon-gamma (IFN-γ) and tumor necrosis factor-α (TNF-α) were determined by ELISA DuoSet R&D Systems.
STATISTICAL ANALYSES
The Statistical Program for Social Sciences (SPSS), version 20.0, was used for statistical analyses. Quantitative and qualitative data were respectively expressed by measures of central tendency/dispersion and simple/relative frequency. Continuous variables were tested for normality of distribution by Kolmogorov-Smirnov test. The differences for these variables were analyzed using the Mann-Whitney U-test or Student's t-test, according to the distribution. The Chi-squared test compared the frequency of individuals in each category. A p-value < 0.05 was considered to be significant.
RESULTS
Ninety individuals were studied, with a mean age of 11.98 (2.72) years, of which 48 (53%) were male. The BMI for age (BMI/i) and sex (z-score) classified 38 (42.2%) participants as obese and 52 (57.7%) as severe obese, being 3.14 (0.86) years the mean (SD) and 2.02 and 8.52 the minimum and maximum values of BMI/i, respectively. Hepatic steatosis was identified in 56 (62.2%) participants, being more frequent in boys than in girls (58.9 versus 41.1%, p > 0.05). Among these, 50 (89.2%) presented grade I steatosis, five (8.92%) presented grade II and only one (1.78%), grade III steatosis. No case of steatohepatitis (NASH) was identified, considering that the levels of transaminases were within the reference values. Blood pressure levels were measured and found within normal limits. Table I shows the biochemical parameters and the inflammatory markers analyzed considering the presence of NAFLD.
Table I Clinical and laboratory characteristics of 90 children and adolescents studied according to the presence of non-alcoholic fatty liver disease

BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma glutamyl transpeptidase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; HOMA-IR: homeostasis model assessment-insulin resistance; hs-CRP: high sensitivity C reactive protein; TNF-α: tumor necrosis factor-α; IFN-γ: interferon gamma. *Values express as mean (SD). ** Values express as median and interquartile.
The inflammatory profile of all study participants considering obesity degrees is shown in Table II. Table III shows the clinical and laboratory characteristics of obese and severe obese patients, considering the presence of NAFLD. Table IV shows that there was a positive and statistically significant correlation between HOMA-IR, insulin and CRP, different from that of IFN-γ, IL-6, and IL-2. BMI/age also influenced the behavior of TNF-α and CRP.
Table II Behavior of inflammatory markers of 90 children and adolescents studied, according to obesity degrees
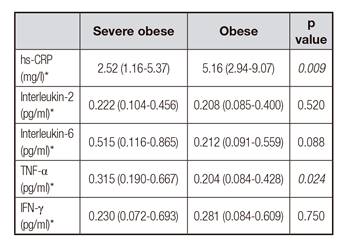
hs-CRP: high sensitivity C reactive protein; TNF-α: tumor necrosis factor-α; IFN-γ: interferon gamma. *Values express as median and interquartiles.
Table III Clinical and laboratory characteristics of 56 children and adolescents with non-alcoholic fatty liver disease according to the obesity degree

BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma glutamyl transpeptidase; LDL: low-density lipoprotein; HDL: high-density lipoprotein; HOMA-IR: homeostasis model assessment-insulin resistance; hs-CRP: high sensitivity C reactive protein; TNF-α: tumor necrosis factor-α; IFN-γ: interferon gamma. * Values express as mean (SD). ** Values express as median and interquartiles.
Table IV Correlation analysis of inflammatory markers and clinical and laboratory parameters of 56 children and adolescents with non-alcoholic fatty liver disease
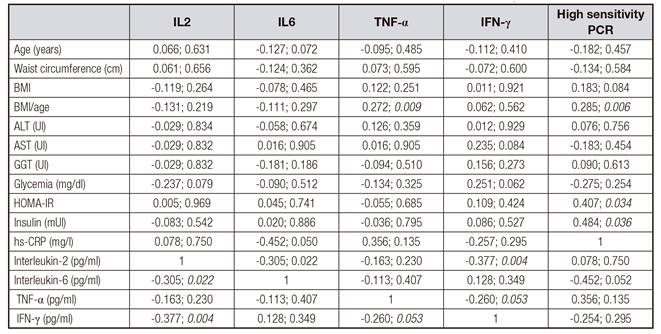
BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma glutamyl transpeptidase; HOMA-IR: homeostasis model assessment-insulin resistance; hs-CRP: C reactive protein-high sensitivity; TNF-α: tumor necrosis factor-α; IFN-γ: interferon gamma.
DISCUSSION
The relevance of this study is due to the fact that the altered findings were from a sample of 90 clinically asymptomatic obese individuals. Hepatic steatosis was prevalent, confirming literature data indicating NAFLD as one of the adverse consequences of obesity, in addition to hyperlipidemia, hypertension and type 2 diabetes mellitus (T2DM) 17. Grade I steatosis was identified in approximately 90% of the 56 children and adolescents diagnosed with NAFLD, reflecting a process of early and incipient installation of the spectrum involving the disease.
In Table I, groups with and without liver disease are compared. We observed that individuals with NAFLD did not present significant differences in the analyzed parameters. However, in Table II, inflammatory markers such as adipokine TNF-α were elevated in severe obese individuals, whereas CRP, an acute phase protein of inflammation secreted by the liver when stimulated by IL-6, was increased in obese individuals, suggesting the presence of an inflammatory state underlying obesity.
In addition, in Table III, the 56 individuals diagnosed with hepatic steatosis were analyzed considering the obesity degree, and statistically significant changes were found. In severe obese patients, waist circumference was higher, probably due to the central distribution pattern of adiposity secondary to insulin resistance. Likewise, severe obese individuals presented the highest rates of GGT. In their study, Fishbein et al. 18 examined the relationship of transaminases with the severity of steatosis and concluded that major changes are expected in severe cases of fatty liver, as opposed to our findings, given the prevalence of steatosis in the early stage has been similar between obese and severe obese. No association between the inflammatory profile and serum GGT values was observed.
As previously mentioned, insulin resistance (IR) is the main determinant of the pathophysiology of NAFDL 8. We observed that the IR indicators (HOMA-IR and serum insulin) were higher in obese patients with NAFLD, but did not differ between patients with and without liver disease. In parallel, TNF-α, an inflammatory cytokine associated with obesity and insulin resistance, showed similar behavior. TNF-α plays a crucial role in the determination of insulin resistance 19, stimulates the synthesis of other cytokines and has direct action in the liver by the increase of lipogenesis by upregulating catalyzing enzymes 20. In addition, it suppresses the expression of genes involved in the uptake of glucose and fatty acid metabolism favoring its accumulation 21. Finally, the inflammatory cascade triggered by TNF-α would be related to the progression of NAFL to NASH 22. Thus, it can be pointed out that these children and adolescents with NAFLD and severe obesity should be monitored more frequently in the future, since at the time of this study, from the laboratory point of view, there were no indications of hepatitis.
Jovinge et al. 23 report an increase in TNF-α in male subjects related to atherosclerotic disease, although this has not been verified in relation to insulin resistance. However, other studies 24) (25 performed in adults describe a significant correlation between TNF-α, serum insulin and body mass index. In this regard, we evaluated the behavior of TNF-α in cases where HOMA-IR and serum insulin were normal or altered and no statistically significant differences were detected, although this behavior was confirmed when the BMI/age was considered. In addition, a correlation analysis involving the 56 children and adolescents diagnosed with NAFLD was performed. A positive and statistically significant correlation was found between HOMA-IR and serum insulin and CRP, an acute phase protein of inflammation, which has serum levels directly proportional to body mass index and therefore associated with obesity 26. Inversely, the IL-6, an inflammatory cytokine associated with obesity and insulin resistance, secreted by T and B lymphocytes, endothelium, fibroblasts and macrophages, showed a negative and significant correlation with IL2, a cytokine that participates in the immune response, secreted by T lymphocytes, suggesting a modulatory effect between cytokines 27. Thus, in our study we may suggest that changes in inflammatory markers may have influenced glycemic metabolic indicators with respect to insulin resistance and NAFLD in subjects with severe obesity.
Most likely, limitations of the study refer to its cross-sectional design, which because of its characteristics does not make it possible to establish a cause and effect relationship. Likewise, individual immune variations, the complexity involving the characterization of the inflammatory status and the verification of the cytokine dosages in a single moment probably may have compromised the verification of the inflammatory framework intrinsic to obesity. We also emphasize limitations involving the imaging method (ultrasound) used for the diagnosis of NAFLD, which does not allow determining the severity of the inflammation with respect to the presence of steatohepatitis or fibrosis, which would only be allowed through liver biopsy, considered as gold standard.
Studies 28) (29 are carried out in an attempt to elucidate an alleged low-grade inflammatory process underlying obesity, which would account for the expression of associated comorbidities, such as type 2 diabetes mellitus, systemic arterial hypertension, dyslipidemias and fatty liver. However, understanding is neither complete nor sufficient to define whether obesity will lead to an inflammatory picture or whether inflammation will lead to obesity. In our study, no differences in the profile of inflammatory cytokines between patients with and without NALFD were observed. However, the severely obese had higher median levels of TNF-α and IL-6, although the latter was not significant (p = 0.059), showing, however, a tendency for association. Although not statistically significant, the proportion of serious obese children and adolescents without NALFD was significantly higher than those with this change (52.9 vs 35.7%), suggesting that obesity severity is a confounder of this relationship. In fact, we found that, only among patients with DHGNA, the TNF-α profile was higher and significant among the severe obese when compared to obese patients. A possible explanation for this finding corresponds to the greater mass of adipocytes among the severe obese, confirmed by the larger waist circumferences presented by this group. In fact, abdominal adiposity is associated with increased insulin resistance and hyperinsulinism. Thus, these patients would carry a greater amount of pro-inflammatory cytokines. In addition, considering only the severely obese, we observed that this condition was associated with the most suggestive parameter of NAFLD, which is insulin resistance, variable related to the entire spectrum of the disease, from the emerging steatosis to the progression of the disease to cirrhosis and hepatocarcinoma 30.
Thus, our study suggests that obesity is an inflammatory condition in isolation. In some cases, comorbidities such as NAFLD can be expressed. Thus, we suggest the investigation of this population in view of the presumption of an incipient inflammatory condition since the onset of obesity.
Finally, this study intends to establish a theoretical reference, considering that the search in the literature resulted in a greater number of articles of revision when compared to the original articles.














