INTRODUCTION
Multiple sclerosis (MS) is a neurodegenerative, chronic, progressive and inflammatory disease that affects the central nervous system (CNS) 1. Its etiology is partially known and includes immunological, genetic and environmental factors (i.e., nutrition) 2. MS is the most frequent cause of neurological disability in young adults and is characterized by the perivenous infiltration of lymphocytes and macrophages in the cerebral parenchyma resulting in demyelination and axonal damage. Approximately 85% of patients start with a relapsing-remitting (RR) clinical course in which there are exacerbations of neurological deficit associated with an inflammatory demyelinating event lasting at least 24 hours in the absence of fever or infection. This is followed by a recovery period in which there is no progression of the disease and may last for months or years 3.
A higher incidence of MS is found in the northernmost latitudes of the northern and the southern hemispheres compared to southernmost latitudes. Recent evidence shows that feeding patterns strongly influence the geographical distribution of MS. For example, MS is uncommon in Japan. Furthermore, Japanese who move to Hawaii change their eating habits and increase the risk of MS. In the Faroe Islands, the incidence of MS was very low compared to other countries of similar latitude and this increased to double as a consequence of the western influence in the diet. The common dietary factor that might explain the previous exceptions is increased fish consumption combined with reduced consumption of meat products 4. The risk of MS increases in non-Hispanic inhabitants of the United States of America, who consume less fish and more foods high in saturated fats 5. It is also known that a diet with a low proportion of polyunsaturated fats in relation to saturated fats is associated with a higher mortality in MS 6.
Increased levels of interleukin-1 (IL-1) and other proinflammatory cytokines play a role in initiating an inflammatory response 7. Dysregulation of proinflammatory cytokines as well as a decrease in antioxidants such as glutathione 8 causes an increase in oxidative stress (OS) by the excessive production of reactive oxygen (ROS) and nitrogen (RNS) species 9) (10. ROS and RNS are produced mainly by mitochondrion, which under normal physiological conditions are controlled through several antioxidant mechanisms 11.
Mitochondria are spherical or filamentous organelles that are localized in all eukaryotic cells and are the main site where adenosine triphosphate (ATP) is synthesized by ATP synthase 12. ATP synthase is the enzyme that uses adenosine diphosphate (ADP), inorganic phosphate (Pi) and electrochemical gradient of protons through the internal mitochondrial membrane as substrates 12. The enzyme can function as ATP synthase (ATP synthesis) or ATPase (ATP hydrolysis) 12 and is formed by a soluble portion F1 and a F0 portion embedded in the lipid membrane 13. The F1 moiety is rich in phenylalanine and methionine amino acids that are highly susceptible to being oxidized by free radicals 13) (14. This oxidation results in a conformational change of the F1 portion and as a consequence alters both the synthesis and the hydrolysis of ATP 13. Under physiological conditions the F1 portion maintains the conformational change suitable for the production of ATP and under pathological conditions is modified by increasing the hydrolysis of ATP 15.
Recent evidence shows a relationship between OS and mitochondrial dysfunction with inflammation in MS. The inflammatory phenomenon conditions severe mitochondrial dysfunction and axonal damage 16. Derived from the inflammatory process, in acute lesions, the intra-axonal mitochondria are damaged by the excessive production of nitric oxide (NO) and RNS 16. ROS and RNS oxidize the membrane phospholipids, which causes a decrease in the flexibility of their chains, altering membrane fluidity 17. This phenomenon of excessive ROS production could be assessed indirectly by changes in ATPase activity and in the physical properties of the membrane, particularly membrane fluidity.
Current treatments for MS (particularly in Mexico) involve anti-inflammatory and immunomodulatory drugs 18. In the present study, patients receive interferon β (INF-β), a pleiotropic molecule that increases the secretion of interleukins with anti-inflammatory properties (i.e. interleukin 4 [IL-4] and interleukin 10 [IL-10]). In addition, INF-β stabilizes tumor necrosis factor α (TNF-α) levels as well as decreases the production of proinflammatory cytokines (interleukins 1, 6 and 12) 18.
On the other hand, previous studies have shown that the administration of omega-3 fatty acids decreases the production of proinflammatory cytokines and OS 9. In particular, dietary supplementation with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) omega-3 polyunsaturated fatty acids (PUFAs) are able to reduce the production of proinflammatory cytokines such as IL-1, IL-6, kin 8 (IL-8) and TNFα 10. In addition omega-3-PUFAs favors the production of prostaglandins E1 and E2 19, diminish the proliferation of T 19 lymphocytes and proinflammatory cytokines (IL-1β, TNF-α and IL-6) 20. In addition, the intake of omega-3-PUFAs increases the synthesis of pro-resolution molecules such as lipoxins, resolvins and protectins 21. Finally the EPA (an omega-3-PUFAs), is a substrate of cyclooxygenase 1 and 2 (COX-1 and COX-2), and lipoxygenase 5 19. EPA competes for the site of action of these oxygenases with arachidonic acid (AA), the latter being responsible for favoring the synthesis of inflammatory cytokines and leukotrienes in conditions where OS prevails 19.
Olive oil mainly contains oleic acid, a monounsaturated fatty acid and a number of bioactive compounds such as polyphenols. According to Puertollano 2015, oleic acid replaces palmitic acid in phospholipids and dissociates lipid rafts 22. This phenomenon is associated with decreases in the incidence of inflammatory diseases, OS markers and in the production of proinflammatory cytokines 23. There is evidence that a diet rich in monounsaturated fatty acids (i.e. oleic) significantly decreases the amount of the IL-1 adhesion molecule in peripheral blood monocyte cells 24. Similarly, the Mediterranean diet (rich in olive oil) decreases the serum levels of interleukins 18, 7 and 6 23. Finally, it is reported that serum levels of omega-3 and 6-fatty acids are decreased in patients with MS 8.
The objective of this work was to evaluate the mitochondrial membrane fluidity and the hydrolytic activity of ATPase of platelets of patients with RR-MS who received dietary supplementation of fish or olive oil as a support for their treatment of interferon beta-1b over a year.
MATERIALS AND METHODS
STUDY DESIGN
A randomized, double blinded clinical trial was performed in the multiple sclerosis clinic of the Neurology Department, Unidad Médica de Alta Especialidad (UMAE), Hospital de Especialidades (HE), Centro Médico Nacional de Occidente (CMNO), IMSS, Guadalajara, JAL, Mexico. Age of participants was 18-55 years. Patients had clinically definite and magnetic resonance image supported MS, at least one relapse in the year before entry into the study, and a baseline EDSS score of < 5 and were treated with subcutaneous 250 μg interferon beta-1b (Betaseron, Bayer) every other day at least one year before the trial. Interferon beta-1b was routinely administered in the afternoon.
Identification numbers were assigned to assure patient confidentiality. This study was performed according with the updated Declaration of Helsinki and all procedures were approved by the Ethics and Health Research Committee of the Mexican Social Security Institute (clave R-2010-1301-8).
Patients were randomly assigned in a 1:1 ratio to receive oral fish oil (4 g/day: 0.8 g EPA and 1.6 g DHA) or olive oil (1 g oleic acid), with a computer-generated randomization sequence. To ensure masking between the fish oil and olive oil, capsules were identical in appearance, packaging, and labeling. Participants reported daily consumption of the supplement in a consumption posting sheet. The rate of adherence to the treatment was > 80% and was determined by using the following formula:
Adherence to treatment (%) = number of capsules actually taken from the last count/number of tablets should be taken at the same stage x 100%. An independent physician evaluated the EDSS score and collected the samples at each clinical visit. Fasting blood samples were taken at 0, 3, 6, 9, and 12 months.
Patients were excluded if they were taking another supplement; had progressive forms of MS; had chronic-degenerative diseases (i.e. diabetes mellitus, hypertension arterial) had history of acute liver or renal dysfunction; had history of tobacco, drug, or alcohol abuse; had intolerance, contraindication, or allergy to fish oil; and had customary antioxidant intake.
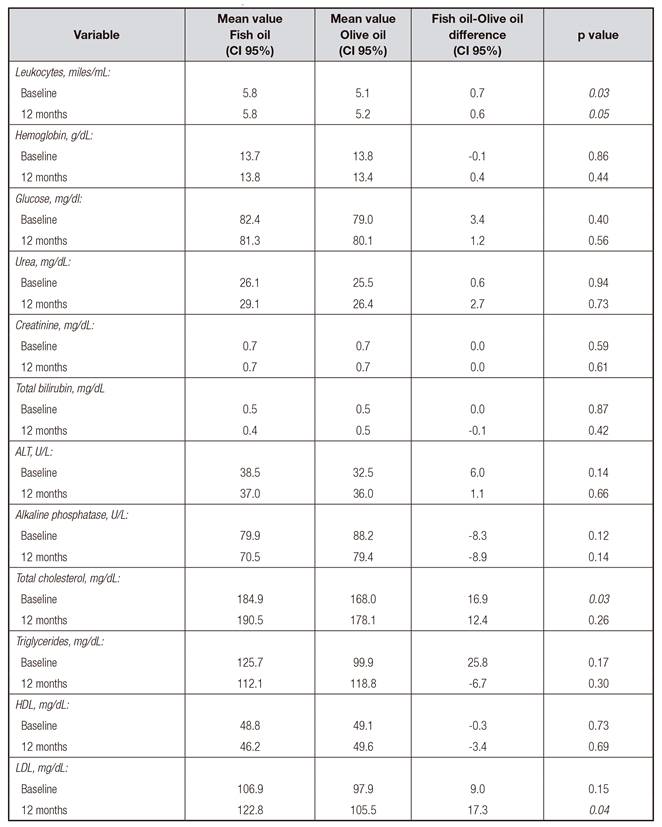
Baseline clinical markers (glucose, cholesterol, triglycerides, total bilirubin and amino transferases are reported in the results section (Table I). The groups of olive oil (O), fish oil (P) and control group (C) were matched by age and sex. To form the control group, 25 clinically healthy individuals were included in order to compare the baseline values of the parameters analyzed in this study.
OUTCOMES MEASUREMENTS
Peripheral venous blood (ten milliliters) was collected in tubes containing etilendiaminotetracetic acid (EDTA) and centrifuged at 310 g for 10 minutes at 4 °C, in order to obtain the plasma and globular concentrate. The plasma was separated and deposited in Eppendorf tubes and centrifuged for 15 minutes at 1,160 g at 4 °C, and the platelet pellet was obtained. The platelet pellet was re-suspended in 200 μl of cold buffer (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM Hepes (pH 7.4) y 0.01% bovine serum albumin. Platelet mitochondrial membranes were obtained as reported elsewhere 25 and were frozen at -80 °C until processing. The protein concentration of the samples was quantified by the Lowry method using bovine serum albumin as standard 26.
The hydrolytic activity of the mitochondrial ATPase was evaluated using a colorimetric method through the liberation of inorganic phosphate using ATPase buffer (125 mM KCl, 40 mM of Mops (pH 8), 3 mM MgCl2). Inorganic phosphate was quantified according to Sumner 27.
Membrane fluidity was estimated from the excimer to monomer fluorescence intensity ratio (Ie/Im) of the fluorescent probe 1,3 dipyrenylpropane (DyPP) incorporated in mitochondrial membranes of platelets as reported previously 28. Fluorescence corrections obtained from readings of membranes without DyPP were applied to all fluorescence values.
STATISTICAL ANALYSIS
Data were analyzed as mean values ± standard deviation. Differences in the parameters studied between groups were evaluated using analysis of variance (ANOVA and MANOVA). A value of p < 0.05 was considered statistically significant. Homogeneity test of variance was performed by Levene test with a 92% result and the Shapiro Wilk test to verify the normal distribution yields a value of 95%. The analyses were done in SPSS version 21.
RESULTS
As shown in Figure 1, of the 300 MS patients treated at the neurology clinic, 50 of them met the inclusion criteria. However, 4 of them dropped out of the study, so 23 patients were assigned per treatment group. The mean age of the patients who participated in this study was 35.1 ± 7.6 years and the baseline EDSS score was 2.1 ± 0.98, after 12 months of the intervention was 2.84 ± 0.94, with a p = 0.79 being non-significant statistically. The evolution of the disease in years is 7.1. Prior to the intervention and at the end of the study, a biochemical safety profile was determined which consisted of blood biometrics, lipid profile, and liver and renal function tests. There was a significant difference between the study groups in the baseline measurements of leukocytes and total cholesterol, being lower in the control group; after 12 months a significant difference was observed in low-density lipoprotein (LDL) (Table I).
BIOCHEMICAL PARAMETERS
Figure 2 shows a significant decrease in the fluidity of the platelet mitochondrial membranes of patients with RR-MS (baseline) compared to the group of clinically healthy individuals (control). At three months of the diet supplemented with fish or olive oil, no significant difference was observed in contrast to that observed at baseline. However, after 6 months of treatment (fish or olive oil, respectively), membrane fluidity in mitochondrial membrane of platelets from patients with RR-MS reached values similar to those of control individuals and remained so until the end of the study.

Figure 2 Mitochondrial membrane fluidity index (Ie/Im) in patients receiving fish oil (F) or olive oil (O) as a dietary supplement, at each experimental time and of individuals in the control group (C). *p ≤ 0.05.
The hydrolytic activity of the mitochondrial ATPase is significantly higher in the patients compared to the individuals in the control group. In addition, the enzymatic activity decreases significantly after 6 months in the olive oil group, in contrast to the basal state. Whereas for the fish oil group the significant decrease of the enzymatic activity is reached after 9 months of treatment. With both treatments, the activity is maintained at levels similar to the control group until the end of the study (Fig. 3).
DISCUSSION
Epidemiological and experimental studies suggest a high prevalence of MS in populations with a high intake of saturated fats, especially of animal origin 29. It has been proposed that consumption of saturated fat causes a change in membrane integrity and functionality 29, which is consistent with the decrease in membrane fluidity in patient's platelets detected in this study. We also observed that the hydrolytic activity of ATPase decreased with the treatments of fish or olive oil in relation to the basal group. These changes are inversely associated with changes in membrane fluidity, i.e. at higher membrane fluidity there is less hydrolytic activity of ATPase.
The fluidity of cell membranes depends on the temperature, the ratio of saturated fatty acids/polyunsaturated fatty acids, the presence of lipid rafts, the proportion of cholesterol present in the membrane, among other factors 30. Our results showed that an appropriate value of membrane fluidity is restored with the consumption of unsaturated fatty acids, whether fish oil or olive oil are given. In the case of the PUFAs present in fish oil, the change in fluidity can be attributed to the incorporation of EPA into the cell membranes. This fact is consistent with the results found in our work group where there was an increase in omega-3-PUFAs levels in patients after treatment with fish oil, in addition to a decrease in levels of AA 8. The recovery of membrane fluidity is accompanied by a decrease in ATPase activity. This would ensure that ATP synthesis activity remains elevated in the mitochondria.
These results provide an encouraging picture of the use of both types of oil; the reduction decrease in the synthesis of inflammatory cytokines (TNFα, IL-1β and IL-6) and NO catabolites in patients receiving oil fish 20. At this regard, it is known that the synthesis of proinflammatory cytokines are derived from the AA cascade, so an increase in EPA and DHA from supplementation is associated with a decrease in proinflammatory cytokines 21. On the other hand, the required ratio of AA/EPA to diminished cellular inflammation is 1.5/3, this ratio increases to 3/6 for moderate cellular inflammation. Consistent with this proposal, we have previously found that the AA/EPA and omega6/omega 3 ratios decreases significantly with fish oil treatment. Although there are significant differences in the lipid profile of the membranes analyzed in the above work 8, the values of our study of membrane fluidity and ATP hydrolysis of the group supplemented with fish or olive oil group are almost similarly. The result described is attributed to the fact that olive oil contains a high proportion of oleic acid 31 and bioactive polyphenols that have anti-inflammatory and antioxidant properties in vitro and in vivo studies 31. This allows us to explain why both groups (fish or olive oil) have similar behavior. The data obtained in this study are consistent with the decrease in markers of inflammation and OS that occur with fish oil consumption in patients with RR-MS. This has repercussions in reestablishing the membrane fluidity to its physiological ranges and the reduction of pathological activity of the ATPase.
In a previous study it's recommended that optimal nutrition should include the consumption of 35% of polyunsaturated fatty acids (with dietary equilibrium omega 3/omega 6) 29. This for an optimal functioning of the blood-brain barrier 29, which avoids the activation of autoreactive CD4+ T cells within the CNS. Therefore, the vicious cycle of the production of proinflammatory cytokines and OS 5 is limited.
At the recommended doses of interferon beta, lymphopenia can occur. However, leukocytes remained within clinically normal range in both treatment groups. Although there are some differences in absolute amounts of leukocytes between treatments, these changes were not significant at the end of the clinical trial in each intervention group. Therefore, the presence of secondary lymphopenia could be not noteworthy. One limitation of this work is that the study time was short to observe changes in the clinical score EDSS.
CONCLUSIONS
In this clinical trial, an increase in fluidity in the mitochondrial membrane was observed over a year of supplementation in the diet of those patients with RR-MS who received fish or olive oil. These results are directly related to a decrease in ATP hydrolysis and are consistent with the antioxidant and anti-inflammatory properties of both, fish oil and olive oil. The results for fluidity mitochondrial membrane and ATP hydrolysis were very similar to the control group.