INTRODUCTION
Head and neck cancer (HNC) constitute the fifth neoplasm in incidence in the world population, being the third most prevalent tumor. In Spain, it causes 19.19 deaths per 100,000 inhabitants per year in men and 1.12 deaths per 100,000 inhabitants per year in women 1. The treatment of HNC using radiotherapy (RT) exclusively or in combination with surgery and/or chemotherapy is increasingly effective, but it is associated with clinically important effects such as mucositis and odynophagia, which can deteriorate nutritional status by hindering swallowing, leading to weight loss and dehydration, hospital admissions and increasing costs. Mucositis secondary to RT or chemoradiotherapy in HNC occurs in 85-100% of treated patients 2. It is the most important acute toxicity, being the main cause of discontinuation of treatment, and may limit the dose of RT. This factor implies a decrease in the probability of tumor control 3, and worsening the prognosis. Of all types of tumors, HNC are those with more evidence that an unplanned disruption has a negative effect on the outcome 4,5,6,7. Therefore, the prevention of mucositis can have a significant impact on improving treatment outcome in patients with HNC 8. Measures against mucositis are extremely important 9, mainly in patients with pain and inflammation.
The Cochrane Library has reviewed the preventive and therapeutic studies of oral mucositis in cancer patients 10,11, concluding that the strength of these measures is variable and that well-designed trials are needed. In the same line, a review focused exclusively on HNC 12 reported that, to date, no intervention is useful by itself and that it would be necessary to combine different ones acting in the distinct phases of mucositis.
Glutamine is the most abundant amino acid in the body, being present in muscles and blood. It is obtained by endogenous synthesis in muscle, and from the diet, in foods with high protein content. Glutamine provides nitrogen to metabolic activities, has a buffer effect that neutralizes acid excess in muscles and participates in the immune-response, and is the main energetic substrate of the epithelial cells of the oral and intestinal mucosa 13. As a non-essential amino acid, the organism can synthesize it from other amino acids, although in situations of metabolic stress it is considered to be "semi-essential". In such situations, such as trauma, infection, neoplasia and cancer treatments, the organism may not be able to synthesize sufficient endogenous glutamine 14,15 to optimally maintain mucosal structure and function. Glutamine is well absorbed in the small intestine. Clinical data suggests that a safe dose of glutamine is 20-40 g/day. The response to glutamine appears to be dose dependent 16. Regarding the efficacy of glutamine studies in the prevention of oral mucositis in HNC, Cerchietti et al. 17 showed in 29 patients that intravenous glutamine reduced the severity of mucositis. In the recent study by Pattanayak et al. 18, performed in 162 patients, glutamine delayed mucositis occurrence and decreased severity significantly.
In the present work, given the little scientific evidence in the prevention of mucositis in HNC along with the importance of this clinical issue, we intend to study the preventive effect of oral supplementation with glutamine in patients with HNC.
MATERIAL AND METHODS
We conducted a prospective, comparative, non-randomized, cohort study to know the efficacy of a preventive intervention with glutamine. The study was approved by the Subcommittees on Health Ethics and Health Research at the University Hospital Virgen del Rocío of Seville.
The inclusion criteria were: HNC squamous cell carcinoma patients, without mucositis and no other prophylactic measures, and older than 18 years. All included patients received radiation therapy with curative intent, 70 Gy by 2 Gy/day, five fractions per week. The different therapeutic regimens were: radiotherapy, radiotherapy plus cisplatin 100 mg/m2 on days 1, 22 and 43 of irradiation, radiotherapy plus cetuximab 400 mg/m2 in the week prior to radiotherapy, followed by 250 mg/m2 weekly during irradiation, induction chemotherapy with cisplatin 100 mg/m2, docetaxel 75 mg/m2 and 5-fluorouracil 1,000 mg/m2 for three cycles every 21 days, followed by chemoradiotherapy.
All patients were referred prior to initiation of any therapeutic scheme to the Nutrition Service for initial assessment and follow-up. Depending on the nutritional status, nutritional support was indicated as needed. Glutamine was given orally at a dose of 10 g every eight hours.
During the follow-up, patients were examined by different specialists in Radiation Oncology to avoid the possible bias of the coordinator of the study. All clinicians used the RTOG/EORTC scales to rate the degree of mucositis and/or odynophagia 19. All patients were examined once a week and, when requested, assessed for symptoms of mucositis and/or odynophagia and supportive treatment was prescribed if necessary.
SAMPLE SIZE
Sample size was calculated based on the presence of mucositis as the main dependent variable. The incidence of mucositis in patients who do not receive glutamine, secondary to radiotherapy or chemoradiotherapy in HNC, is nearly 90% 2. As indicated in the introduction section, in patients with this condition receiving glutamine, the incidence of mucositis is not well established; however, indirect data from one study 17 showed that the incidence of severe mucositis decreased by 33%. Based on this data, and for the calculation of the sample size, it is assumed that in patients with glutamine the incidence of mucositis can decrease by half, which is 16%. With the above parameters, starting from an unpaired study, and assuming an alpha error of 5% and a statistical power of 90%, it was estimated that it was necessary to compare 131 consecutive patients who had not received glutamine with 131 consecutive patients who received glutamine once it was decided to include glutamine in the supportive nutrition in the hospital (Epi Info version 3.5.1).
STATISTICAL ANALYSIS
The results are presented as frequencies (%) for qualitative variables and means with standard deviation or median with ranges for quantitative variables. For the univariate analysis, the statistical comparison of the qualitative variables was performed using the Chi-squared test. For the analysis of continuous variables, the Student's t-test or analysis of variance (ANOVA) when comparing more than two variables were used. To assess the factors independently associated with the appearance of mucositis, a logistic regression model type step by step backwards was used, where factors with a p > 0.05 were excluded. A p < 0.05 was considered as significant. The analysis was performed using the SPSS statistical package (version 14.0, Chicago, IL, USA).
RESULTS
DEMOGRAPHICS AND HABITS
Of the 131 patients who received glutamine, 87% (n = 114) were males and 13% (n = 17) were females. Regarding the 131 patients who did not receive glutamine, 84% (n = 110) were males and 16% (n = 21) were females. The age was 60.72 ± 11.51 years and 58.52 ± 11.72 years in those receiving or not receiving glutamine, respectively. In terms of anthropometric data, the heights, weights and body mass index were 166.4 ± 7.5 cm, 72.1 ± 17.1 kg and 25.8 ± 5.4 kg/m2 vs 167.4 ± 8.3 cm, 72.5 ± 15 kg and 26.5 ± 4.4 kg/m2 in those receiving or not receiving glutamine, respectively. Tobacco smokers and alcoholic drinkers represented 90.8% (n = 119) and 58% (n = 76) vs 84% (n = 110) and 57.3% (n = 75) of those who received glutamine vs those who did not receive it.
HEAD-AND-NECK TUMOR CHARACTERISTICS AND TREATMENTS
The locations of neoplasms as a function of whether they received glutamine are detailed in Table I. Table II shows the distribution by stages of neoplasia in patients who received and did not receive glutamine. There were no differences in these variables between those patients receiving or not receiving glutamine supplementation.
Table I Locations of head-and-neck tumors in the groups with and without glutamine
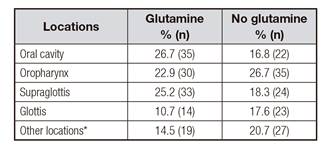
* Nasopharynx, hypopharynx, paranasal sinuses.
Table III indicates the different therapeutic schemes for HNC. In both groups, 100% of patients received radiotherapy, until reaching a dose of 70 Gy, achieving all of them the prescribed radiotherapy dosage. In both groups, glutamine and non-glutamine, the frequency of surgical interventions was equal, 27.5% (n = 36); radiotherapy was given with a complementary intention to surgery in these cases. In the other 72.5% (n = 95) radiotherapy was indicated with radical intention. As for chemotherapy, it was prescribed in 58.8% (n = 77) of patients with glutamine vs 59.5% (n = 78) of patients without glutamine. There were no differences in the types of treatment between both groups.
ANALYSIS OF FACTORS ASSOCIATED TO THE INCIDENCE AND SEVERITY OF MUCOSITIS AND ODYNOPHAGIA
Overall, mucositis was present in 50.4% (n = 66) of patients of the glutamine group and in 59.5% (n = 78) of those who did not receive it (p = 0.14). Among patients receiving glutamine, the mean doses were 0.42 g/kg and 0.43 g/kg in those who developed mucositis vs those who did not (p = 0.55). Regarding the tumor localization, mucositis incidence was higher in those without glutamine supplementation in the oropharynx disease vs those with glutamine (Table IV). Odynophagia incidence and severity were more common in oropharynx and supraglottis diseases in patients without glutamine supplementation vs those with glutamine (Table V and Table VI).
Table IV Mucositis incidence in different tumor localizations regarding the presence or not of glutamine supplementation (univariate analysis)

Table V Odynophagia incidence in different tumor localizations regarding the presence or not of glutamine supplementation (univariate analysis)

Table VI Odynophagia grades III-IV in different tumor localizations regarding the presence or not of glutamine supplementation (univariate analysis)

In the multivariate analysis to identify the variables associated to the presence of mucositis, age, sex, body mass index, smoking and alcohol drinking habits, tumor localizations, stages, chemotherapy, and glutamine supplementation were included. Two variables were associated to the incidence of mucositis: tumor localization and glutamine supplementation. Regarding tumor localization, the supraglottis (risk ratio [RR] and 95% CI: 0.12 [0.05-0.27]), glottis (RR and 95% CI: 0.05 [0.02-0.15]) or hypopharyngeal (RR and 95% CI: 0.08 [0.02-0.27]) diseases were associated with lower mucositis than the disease in the oral cavity (< 0.001). Patients without glutamine had a mucositis RR of 1.78 (95% CI: 1.01-3.16; p = 0.047) compared with those receiving glutamine. In terms of severity, grade I-II mucositis had 35.1% (n = 46) of patients with glutamine vs 40.5% (n = 53) of those without glutamine, and grade III-IV, 15.3% (n = 20) vs 19.1% (n = 25), respectively (p = 0.32). In the multivariate analysis to identify factors associated to the severity of mucositis, no independent variables among those patients with mucositis were found.
Odynophagia was present in 55.7% (n = 73) of patients with glutamine and in 77.9% (n = 102) of those without it (p = 0.0001). Regarding those with glutamine, the mean dose was not different (p = 0.34) among patients who did not have odynophagia (0.44 g/kg) vs those who did (0.42 g/kg). In the multivariate analysis, factors independently associated to odynophagia were glutamine supplementation, chemotherapy and tumor localization. Regarding localization, supraglottis (RR and 95% CI: 5.18 [2.21-12.84]; p = 0.0002), glottis (RR and 95% CI: 7.53 [2.64-24.28]; p = 0.003) and hypopharynx (RR and 95% CI: 4.14 [1.2-17.2]; p = 0.03) diseases were associated with greater odynophagia than the disease of the oral cavity. Patients who did not receive glutamine had a RR of having odynophagia of 2.87 (95% CI: 1.62-5.18; p = 0.0003). Patients who did not require chemotherapy had lower odynophagia incidence (RR 0.4, 95% CI: 0.22-0.73; p = 0.003). As for severity, odynophagia grade I-II had the 43.5% (n = 57) of patients with glutamine vs 35.1% (n = 46) of those without it, and odynophagia grade III-IV had 12.2% (n = 16) of the glutamine group vs 42.7% (n = 56) in patients without glutamine (p < 0.0001). In the multivariate analysis of factors associated to the severity of odynophagia, only patients with odynophagia were included; the RR for grade III-IV odynophagia was 4.33 (95% CI: 2.24-8.74; p < 0.0001) in those who did not receive glutamine vs those with glutamine.
In respect of other clinical features, 6.9% (n = 9) of patients receiving glutamine discontinued oncological treatment as a result of mucositis and/or odynophagia vs 19.8% (n = 26) of patients who did not receive it (p = 0.002). In addition, 77.9% (n = 102) of patients with glutamine required analgesia vs 87.8% (n = 115) without it (p = 0.03). Finally, regarding the need for a nasogastric tube, it was indicated in 3.1% (n = 4) of patients with glutamine and in 9.9% (n = 13) of patients without glutamine (p = 0.02).
DISCUSSION
The present study shows that the supplementation of oral nutrition with glutamine, at a dose of 10 g every eight hours, until reaching a total daily dose of 30 g, reduced the incidence of oral mucositis in patients with chemoradiotherapy because of HNC. None of the other variables included in the multivariate analysis, such as age (less or more of 65 years old), smoking or alcoholic drinking habits, body mass index as an expression of nutrition, TNM classification, chemotherapy and sex were associated with the presence of mucositis during the treatment. Moreover, the present study analyzed odynophagia, since it has an important impact on the quality of life of patients with HNC 20,21 and besides, this clinical feature has not been well analyzed previously 17,18. In the multivariate analysis, the glutamine supplementation was associated with less odynophagia incidence, such as the absence of chemotherapy; in addition, patients with oral cancer had less odynophagia than those with other tumor localizations. Regarding odynophagia severity, the presence of grades III-IV was significantly decreased in patients receiving glutamine. Patients with oropharynx and supraglottis diseases were those with greater improvement after taking glutamine, in terms of mucositis and odynophagia incidences and odynophagia severity. However, this data should be confirmed by a multivariate analysis, which requires a larger sample size in each tumor location.
With respect to the dose of glutamine that the patients should receive, the present results suggest 10 g every eight hours as adequate, reaching a total daily dose of 30 g. Since there were no differences among the patients taking glutamine, in incidence of mucositis and odynophagia in relation to the mean dose in g/kg of weight, it seems that the response is not dose dependent. Nevertheless, this statement must be taken with caution since it is not based on a multivariate analysis.
Other findings of the present study seem relevant, such as the incidence of treatment interruptions because of mucositis and/or odynophagia and the impact on the requirements of analgesia and nasogastric tube. The incidence of treatment discontinuation was significantly lower among patients taking glutamine. Patients with glutamine required significantly fewer prescriptions of analgesia. Also of great interest, being an expression of mucositis and/or severe odynophagia, was the analysis of the use of nasogastric tube, which was significantly lower in patients with glutamine. Based on the reduced need for analgesia and nasogastric tube in patients with glutamine, we believe that a possible added advantage would be a reduction of other economic costs in the treatment of these patients. This statement would require another type of analysis and must be taken with caution.
A strong point in the present study is that the patients included are representative of the normal population of HNC, in terms of age, risk factors and location 22. In addition, other factors merit being highlighted, because they were present with the same frequencies in both groups of patients. The antineoplastic treatments received by patients with glutamine and without glutamine supplementation showed no differences. Thus, in both groups, the frequency of surgery was the same. In both groups, all patients received 70 Gy, ending all cases with the planned radiation therapy. Regarding the indication of chemotherapy, 58.8% and 59.5% of patients with and without glutamine, respectively, received it.
The present study has some limitations, especially that it is a non-randomized study in which patients were included before and after the glutamine use as an amino acid supplementation in the nutritional support of patients with HNC in our institution. However, the sample size of 262 patients, 131 without and 131 with glutamine, was calculated to have enough statistical power, as previously explained.
CONCLUSIONS
Oral glutamine supplementation prevents the incidence of oral mucositis and the incidence and severity of odynophagia secondary to antineoplastic treatment in patients with HNC. In addition, it produces less interruption of treatments, together with a lower requirement of analgesia and nasogastric tube. Therefore, these results suggest that glutamine is deserving of future studies, to confirm the exposed points, by conducting randomized double-blind clinical trials. If these results are confirmed, the use of glutamine should be included in treatment protocols in patients with HNC.
















