INTRODUCTION
Home parenteral nutrition (HPN) is the primary treatment in patients with chronic intestinal failure (CIF), in which intravenous supplementation is required to maintain health and/or growth. HPN is a vital therapy for these patients 1.
A most important concern about HPN is the central venous catheter (CVC) complications, particularly CVC-related infections, which involve high morbidity, mortality and cost for the healthcare system. Numbers are not negligible. In a systematic review of 39 studies, the catheter-related bloodstream infection (CRBSI) rate ranged between 0.38 and 4.58 episodes/1,000 catheter days 2. In our institution, a report of 26 years of experience in HPN showed a CRBSI rate of 2.68 episodes/1,000 catheter days 3.
The origin of catheter-related infections may have different routes. Extraluminal dissemination, by migration of skin organism at the insertion site; intraluminal dissemination, by direct contamination of the catheter hub, the most common route for long-term catheters; and rarely by hematogenous dissemination or by contamination of parenteral nutrition infusion 4. Microorganisms rapidly colonize the CVC. When the microorganism adheres to the intraluminal surface of the catheter, it is included in an extracellular matrix of polymeric substances (exopolysaccharides, fibrin, nucleic acids) that make up the biofilm. This biofilm has many benefits for bacteria survival since it is a defensive barrier against host's immunity and also against antibiotics, and it allows genetic material exchange between microorganisms 5.
Many factors have been associated with the risk of CRBSI. The most important factor to prevent those infections is the existence of a multidisciplinary team providing education and training in HPN and a specific protocol about catheter care with appropriate aseptic measures. The characteristics of the CVC that confer a lower risk are tunneled catheters, with a single lumen, made with silicone or polyurethane materials. On the other hand, there are factors that involve a greater risk of infection: the longer duration of the indwelling catheter, patient's characteristics such us extreme age, immunosuppression, serious disease, loss of skin integrity, the presence of fistula or drainages and HPN-related hyperglycemia 6.
ESPEN guidelines on CIF recommend prevention of CRBSI with a high grade of evidence: education, hand hygiene and aseptic technique of CVC with the use of chlorhexidine 2%, use of tunneled single-lumen catheters and regular change of intravenous administration sets and with a low grade of evidence: catheter locking with taurolidine. In addition, ESPEN recommends re-education and antimicrobial catheter lock in patients who repeatedly present CRBSI. It is not recommended the use of in-line filters, routine replacement of catheters, antibiotic prophylaxis and heparin lock 7.
Taurolidine is a non-antibiotic, antimicrobial agent, derived from the aminosulphonic acid tauramide and formaldehyde. It metabolizes into taurine, carbon dioxide and water. Taurolidine presents a broad spectrum against Gram-positive, Gram-negative bacteria, also including methicillin-and vancomycin-resistant bacteria (MRSA, VISA and VRE) and fungi 8,9. Its mechanism of action is thought to be irreversible binding to the cell walls of organisms, preventing their adhesion to the biological surfaces, the colonization and, therefore, the biofilm formation 10. There have not been any reports on microbial adaptation 11 or toxic effects.
Taurolidine lock has demonstrated efficacy in the prevention of CVC-related infection in patients on hemodialysis 12 and chemotheraphy 13. In the last years, taurolidine lock solution has been used on HPN and several studies have suggested a reduction in CRBSI rate in adult 14,15 and pediatric population 16.
In the present study, we reviewed patients on HPN in our hospital with taurolidine lock solution. We compared the CRBSI rates before and during this intervention and its cost-effectiveness.
MATERIALS AND METHODS
A retrospective study was performed reviewing patients on HPN in our institution (Nutrition Unit, Hospital Gregorio Marañón) treated with taurolidine lock from June 1995 to April 2017. The taurolidine lock was initiated in July 2013.
According to our protocol, patients eligible for HPN are admitted before the initiation of the treatment and trained on aseptic technique by a specialized multidisciplinary team. CVCs are implanted under aseptic conditions and with radiologic and ultrasound guidance. Patients are instructed to flush the catheter with saline after each use to maintain the patency. CRBSI rate is a quality indicator in our unit, so patients are closely followed-up and receive re-education after any new catheter infection.
Our criteria to prescribe taurolidine involve patients with a high CRBSI rate defining as CRBSI rate > 2 episodes per 1,000 catheter days or with a recent CRBSI to attempt to save the catheter. Each patient acted as his/her own control. Informed consent was obtained for all participants.
These patients were instructed to instill 3 ml of taurolidine 2% (TauroSept®, Geistlich) into their CVC after infusing each parenteral nutrition bag. Taurolidine remained in situ without being aspirated before the next parenteral nutrition infusion.
We extracted from our medical records the following data: patient characteristics (age, gender, race, underlying disease and indication for initiating HPN), incidence of catheter-related infections, microbiology, number of catheter days, number of line exchanges, type of catheter, incidence of catheter-noninfectious complications, number, calories and components of parenteral nutrition bags and hospital admissions data due to CRBSI.
We considered a CRBSI when there was an isolation of the same organism for semi-quantitative or quantitative cultures of both blood drawn from the catheter lumen and blood drawn from a peripheral vein of a patient with clinical symptoms of bloodstream infection (fever, chills, elevated white blood cell count, etc.) 2. CRBSI treatment included hospital admission and intravenous systemic antibiotic or antifungal, antibiotic lock according to the results of blood cultures and catheter removal if necessary. Catheter occlusion was defined as either impossibility of infusing any fluid or drawing blood through the catheter or need to exert an excessive pressure or both. Treatment included fibrinolytic agents infusion and catheter removal in some cases. CRBSI and occlusion rates were expressed as the number of catheter related episodes per 1,000 catheter days and were grouped into pre-intervention (without taurolidine) and per-intervention group (with taurolidine).
To evaluate the cost-effectiveness of this intervention, in the first period the number of hospital admissions due to CRBSI and catheter removals due to infections and vascular occlusions on HPN patients were counted. In the second period, the cost of taurolidine locks was added. We used the DRG (Diagnosis-Related Group). The DRG uses demographic variables and diagnosis criteria to classify patients into groups that can be compared clinically, with similar inpatient durations and resources consumption 17. Therefore, the treatment costs for the cases included in each DRG should be similar. For the cost of hospital admission due to CRBSI we used the cost of DRG 452 (treatment complications with major complication), with a price of 3,560.40 euros/episode 18. Insertion of CVC has an estimated cost of 614.19 euros/catheter 19. The price of TauroSept® (Geistlich) lock is 2 euros per use.
In the descriptive analysis, the patients' characteristics are presented as numbers (n), frequencies (%) and mean with standard deviation. CRBSI and catheter occlusion are expressed as rates (episodes per 1,000 catheter days) with their confidence interval (CI 95%). A value of p < 0.05 was considered as statistically significant. Statistical analysis was performed using Epidat® 3.1.
RESULTS
Thirteen patients, six (46%) males and seven (54%) females, received taurolidine lock. The mean age at initiation of taurolidine was 61.08 (SD = 14.18) years. The underlying disease was benign in five patients (38.5%) and malignant in eight (61.5%). The benign diseases were three inflammatory bowel diseases and two adherence syndromes after peritonitis. The malignant diseases were a leiomyosarcoma, retroperitoneal liposarcoma, pancreatic, colon, rectal, ovarian and two cervical cancers. Main indications of HPN were short bowel syndrome (n = 7), intestinal fistula (n = 1), mechanical obstruction (n = 1), intestinal dysmotility (n = 1) and extensive small bowel mucosal disease (n = 3). In all patients, the HPN was complementary to oral diet/enteral nutrition.
Twelve patients had a single lumen tunneled Hickman® catheter in the jugular vein. A peripherally inserted central catheter (PPIC) was initially used in one patient but was switched to a single lumen tunneled Hickman® catheter three months later. Only three patients initiated taurolidine with a new CVC.
In nine patients (69.2%) taurolidine was prescribed due to a high CRBSI rate, whereas in four patients (30.8%) the indication was to attempt to save the CVC after a recent CRBSI (Table 1).
The total days of catheterization pre and per-taurolidine were 12,186 and 5,293, respectively. There were 38 episodes of CRBSI in the first period without taurolidine vs four episodes in the second period without taurolidine. The CRBSI rate pre vs per-taurolidine was 3.12 (range 1.08-23.81) vs 0.76 (range 0-3.21) episodes per 1,000 catheter days (p = 0.0058). Incidence ratios of taurolidine compared to saline were 0.24 (95% CI, 0.09-0.68). A total of ten patients (76.9%) did not have any other CRBSI after taurolidine lock.
Even excluding those patients with a longer pre-taurolidine period, the results were very similar. On this occasion, the days and CRBSI rate pre and per-taurolidine period were 4,905 and 4.89 vs 5,074 days and 0.79 episodes per 1,000 catheter days (p = 0.0001), respectively.
The CRBSI rate before taurolidine lock in patients with a benign vs malignant underlying disease was 2.21 vs 7.15 episodes per 1,000 catheter days (p < 0.001). Nevertheless, this difference was not significant (p = 0.66) during taurolidine lock, with a CRBSI rate of 1.27 for benign disease vs no episodes in malignant disease. Furthermore, there was not a significant difference (p = 0.31) in CRBSI rate for benign underlying diseases in pre and per-taurolidine period, 2.21 vs 1.27; however, there was a significant difference between pre and per-taurolidine intervention in patients with malignant underlying diseases, 7.15 vs no episodes, respectively (p < 0.001).
According to the indication, for those who attempted to save the catheter after a recent CRBSI, the CRBSI rate pre and per-taurolidine was 1.74 vs 1.1 episodes per 1,000 catheter days (p = 0.31), whereas when the indication was a high CRBSI rate, the CRBSI rate was 9.72 vs 0.39, respectively (p < 0.001).
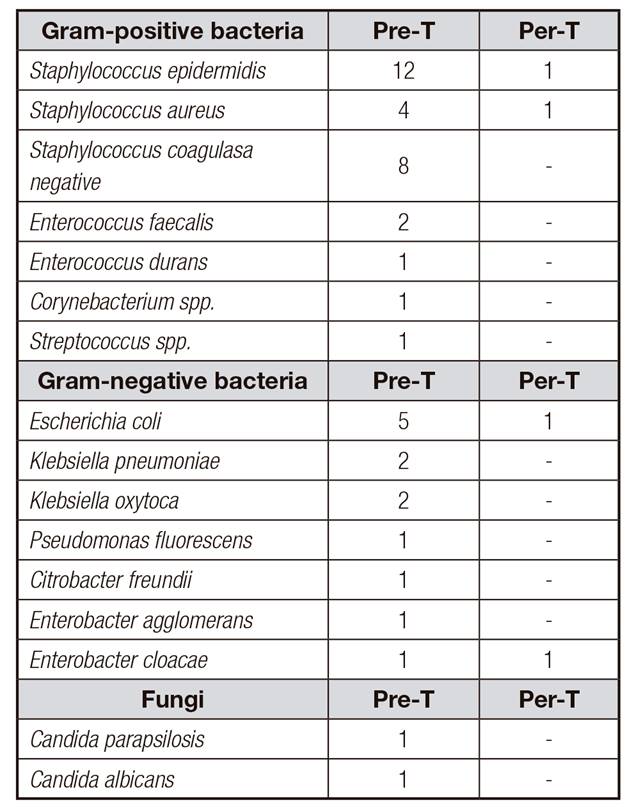
In the pre-taurolidine period, the microorganisms identified were 55.3% Gram-positive bacteria, 28.9% Gram-negative bacteria, 5.3% fungi and 10.5% polymicrobial. During taurolidine lock, there were 50% of Gram-positive and 50% Gram-negative bacteria. Pathogens identified were described above (Table 2).
There were differences in CRBSI rates caused by Gram-positive bacteria before and during taurolidine lock (Fig. 1).
Pre-intervention, in 22 out of 38 CRBSI the catheter had to be removed. Responsible pathogens were Staphylococcus epidermidis (n = 7), Staphylococcus aureus (n = 3), Klebsiella oxytoca (n = 3), Escherichia coli (n = 2), Staphylococcus coagulase negative (n = 1), Candida albicans (n = 1), Candida parapsilosis (n = 1), Pseudomonas fluorescens (n = 1), Enterobacter cloacae (n = 1) and polymicrobial (n = 2). Per-intervention, catheter was withdrawn in four out of four CRBSI. The identified organism were Staphylococcus epidermidis (n = 1), Staphylococcus aureus (n = 1), Escherichia coli (n = 1) and Enterobacter cloacae (n = 1).
Five patients received anticoagulant agents, two were on acenocoumarol whereas three were on low-molecular-weight-heparin. Catheter removal related occlusions reached four before taurolidine an only one after taurolidine. Occlusion rates were 0.33 vs 0.19 per 1,000 catheters days respectively, difference not significant (p = 0.34). Incidence ratio of taurolidine compared to saline for occlusions was 0.58 (95% CI, 0.01-5.82). In all cases of occlusion, CVC was removed.
None of the patients reported any adverse effects with the taurolidine lock solution.
The total cost in the pre-taurolidine period due to CRBSI hospital admission and catheter removals was 151,264.14 euros vs 24,331.19 euros in the pre-taurolidine period, including the cost of the taurolidine lock solution. The average per-patient cost was 11,635.70 euros vs 1,871.63 euros without and with the use of taurolidine, respectively. This means a daily cost of 12.4 euros/day before taurolidine lock vs 4.6 euros/day after its use, with a mean of 4.92 bags of HPN per week (Table 3).
DISCUSSION
CRBSI are the most frequent complications in patients on HPN and have important impact in the cost of the treatment. Moreover, CRBSI are rated as one of the first outcome indicators among these patients 20. Our study evidences a substantial reduction in CRBSI rate with the taurolidine lock and great economic benefit with this intervention.
We found an important decrease in CRBSI rate with taurolidine lock from 3.12 to 0.76 episodes per 1,000 catheter days (p = 0.0058). These results are in agreement with previous reports 14,15,21,22,23,24.
We observed a drastic reduction of the CRBSI rate when the indication for the taurolidine lock was a high CRBSI rate, which was pre and per-taurolidine 9.72 vs 0.39 episodes per 1,000 catheter days (p < 0.001). However, when the indication was to attempt to save the catheter for a recent CRBSI, although the individual CRBSI rate was less than two episodes per 1,000 catheters days, no significant differences between the two periods were found. This results support the benefit of using the taurolidine lock in selected patients, as those with a high CRBSI rate, as some authors had suggested 25. Saunders et al. went further and suggested that taurolidine lock reduces CRBSI in high-risk subgroups of patients. The proposed the Southampton criteria for the taurolidine use in HPN patients defining as: two or more episodes of CRBSI acquired in the community in a period of 12 months, two or more episodes of CRBSI community or hospital acquired in patients with a potential persistent source of intra-abdominal sepsis and patients with a high-risk vascular access 26. Considering these observations, taurolidine may be an effective locking in patients with high risk of CRBSI not only measured by rates but also considering some patients' characteristics. According to the previous information, our results suggest that taurolidine lock is an effective intervention especially in patients with underlying malignant disease, both palliative and curative.
Additionally, it is encouraging that ten out of 13 patients did not present any further CRBSI after taurolidine locking. The pathogens identified in these CRBSI were Escherichia coli, Enterobacter cloacae, methicillin-sensitive Staphylococcus aureus (MASSA) and Staphylococcus epidermidis, and no resistance has been reported with taurolidine, until now 11. The underlying disease for these three patients that presented a CRBSI in the per-taurolidine period was, surprisingly, a being condition, with two cases of an active Crohn's disease and a case of common variable immunodeficiency. This finding could support that in an underlying immunodeficiency taurolidine lock may not be as effective, as suggested in a previous study 16.
In general, in our series, the CRBSI rate is in agreement with the literature and the predominant organism are the same as well 2. The pathogens implicated in the infections were similar in both periods, except for the absence of fungi infections in per-taurolidine period. However, only four cases of CRBSI during the taurolidine period are too little to assess appropriately changes in the responsible pathogens.
In addition, there were less vascular occlusions in the per-taurolidine period compared to the pre-taurolidine intervention, 0.19 vs 0.33 per 1000 catheter days, respectively, as other study showed 27, but without a significant difference, in part, due to the low incidence of occlusion rates by decreasing infections. A meta-analysis did not observe differences in the occlusions incidence rates, maybe because of the heterogeneity of the studies included 23. This decrease in vascular occlusions may be explained by a promotion of intraluminal hemodynamics by reducing local pathological coagulation phenomena. Taurolidine causes inhibition of staphylo-coagulase-mediated coagulation which cannot be influenced by heparin. The risk of pathological staphylo-coagulase-induced coagulation occurring especially at the CVC tip is therefore reduced 28. Reliability in long term use is due to the absence of development of microbial resistance due to its condition of antimicrobial that interacts directly with the cell wall structures. On the other hand, studies with ethanol lock have showed an increase of vascular occlusions 29.
Nowadays, there are still some issues about the taurolidine lock, such as frequency of use, type of solution, method of use, etc. We decided to instill taurolidine after each parenteral nutrition because it has been observed that, although taurolidine is effective both instilled one a week or after each parenteral nutrition, there is a greater decrease in the incidence of CRBSI when using it after each infusion 22. There are different taurolidine solutions, which contain taurolidine 2% or 1.34%, and could be isolated or accompanied with citrate or heparin. We decided to use taurolidine 2% for its greater evidenced power in in vitro studies 9 and we chose the isolated solution since it has been described that heparin and low levels of citrate may promote biofilm formation 30.
In the present study, no adverse events occurred. Nevertheless, Olthof et al. 27 reported side effects that urged to stop taurolidine or switch to a different taurolidine formulation or to saline. The most frequent were mild side effects such as burning sensation, dizziness, nausea or pain and paresthesia, palpitations or discomfort, but also some cases of an anaphylactic-like reaction.
Finally, our study evidences that taurolidine lock is a cost-effective intervention. The total cost due to CRBSI hospital admissions and catheter removals before the taurolidine lock was 151,264.14 euros and 12.4 euros per day and after adding the taurolidine lock solution the cost was 24,331.19 euros and 4.6 euros per day. This shows that taurolidine is a money-saving intervention, interesting for the healthcare system.
The major limitations in the current study are related to the retrospective design and with the small sample of patients. Despite the limited number of patients studied (n = 13), the periods analyzed in each group were considerably large (12,186 and 5,293 days respectively). The study was not randomized but all patients received the same information about catheter care and no other treatments. Moreover, the results should be interpreted with caution. Possible confusion factors may play a role in the cause of CRBSI, however, we could not study them since the number of patients included was too low to perform a multivariable analysis.
In conclusion, our study shows that taurolidine lock is a cost-effective intervention in patients on HPN with high risk of infections without causing an increase in catheter occlusion rates. According to our data, taurolidine lock should be prioritized in patients with underlying malignant disease and/or a high rate of CRBSI.
Before long, the results of a multicenter randomized controlled trial aimed to assess the effectiveness of taurolidine and saline in CRBSI prevention (ClinicalTrials.gov Identifier NCT01826526) may give more light in this field.