INTRODUCTION
Patients with systemic inflammatory responses experience intense catabolism that aggravates their clinical and nutritional status, and the consequences of these events are high complication and mortality rates 1,2). The optimization of intravenous fluid management and nutritional intake is fundamental in the care of critically ill patients. The objective of intravenous fluid therapy is to maintain adequate intravascular volume and prevent tissue hypoperfusion and organ dysfunction 3,4. However, continued and repeated infusion of fluids results in edema, electrolyte imbalance, and inadequate weight gain, all of which contribute to worse outcomes including increase of mortality 5,6-7. In addition, patients also receive fluids through diluents, medications, nutrition, and maintenance fluids 8. Therefore, similar to other medications, fluids have qualitative and quantitative adverse effects 9,10.
Strategies to prevent fluid overload are beneficial after hemodynamic stability 11. Enteral nutrition is one of the recommended methods for feeding critically ill patients 12. Enteral nutrition provision and fluid therapy should be balanced as continuous fluid infusion may lead to edema of the mesentery and intestinal wall, which results in dysmotility, vomiting, and ileus, all of which compromise the provision of enteral nutrition 13. According to the guidelines of the Surviving Sepsis Campaign 14, patients with septic shock should receive fluid resuscitation with an initial administration of 30 ml of fluid/kg of body weight. In line with this recommendation, the British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients suggests a more restrictive fluid volume 15,16.
Because no study has assessed the relationship between fluid administration and enteral nutrition infusion, the present study aimed to evaluate the effect of intravenous fluid administration on caloric and protein deficits in critically ill patients on mechanical ventilation and exclusively receiving enteral nutrition.
MATERIAL AND METHODS
This prospective cohort study was conducted between June 2014 and December 2015 in a tertiary hospital. The study was approved by the Human Research Ethics Committee of the Júlio Müller Hospital and was conducted according to the 1975 Declaration of Helsinki, revised in 2000. The relatives or guardians of the included patients provided informed consent, if the own patient could not sign the term.
INCLUSION AND EXCLUSION CRITERIA
The study included consecutive critically ill non-surgical patients who were admitted for a minimum of five days for clinical treatment, who were on mechanical ventilation in the intensive care unit (ICU), and who received exclusive enteral nutrition. The study excluded pregnant patients, patients with late introduction to enteral feeding (> 48 h after admission), patients who received parenteral nutrition associated or not to enteral nutrition, and patients who died within the first five days of hospitalization.
INSTITUTIONAL NUTRITIONAL THERAPY PROTOCOL
Enteral feeding was initiated within 24 h after hemodynamic stability was reached and after the confirmation of the feeding tube position in the X-ray. Caloric and protein requirements were calculated according to the guidelines from the European Society for Parenteral and Enteral Nutrition 12. The targets were 25-30 kcal/kg of body weight and 1.25-2.0 g of protein/kg of body weight. The goal was to reach the targets on the third or fourth day of the nutrition regimen. To achieve this goal, either one-third or one-fourth of the calculated daily enteral feeding requirements was prescribed per day.
DEMOGRAPHIC, CLINICAL, NUTRITIONAL, BIOCHEMICAL AND MORTALITY-RELATED DATA
Age, sex, body weight, and nutritional status during the first 24 h of hospitalization were recorded. The illness severity score was determined using the Simplified Acute Physiology Score 3 (SAPS 3) in the ICU. The five-day caloric and protein requirements and deficits were calculated. Levels of C-reactive protein (mg/l), albumin (g/dl), lactate (mg/dl), and serum glucose (mg/dl) were measured daily. The serum albumin level was dichotomized into ≥ 3.5 g/dl or < 3.5 g/dl. Mortality at 28 days and length of hospital stay were recorded. Each patient's nutritional status was evaluated using the Subjective Global Assessment (SGA), which divides patients into the categories of SGA A (eutrophic), SGA B (at risk of malnutrition or moderate malnutrition), or SGA C (severe malnutrition).
CALORIC AND PROTEIN DEFICITS
Caloric and protein deficits were calculated on the fifth day of hospitalization. These calculations determined the difference between the prescribed amounts of calories and protein and the amount received over the course of the five days. All prescriptions of calories and protein followed the guidelines cited above 12). For statistical analysis, the patients were divided into two groups according to caloric and protein deficits: a) those with a caloric deficit (i.e., ≥ 480 kcal/day) and/or with a critical protein deficit (i.e., ≥ 20 g of protein/day); and b) those with no caloric deficit and/or critical protein deficit (deficits which did not reach the aforementioned levels). These parameters were based on a previous study 17 that showed that these limits of caloric and protein deficits are significantly sensitive for predicting the risk of mortality.
VOLUME OF INTRAVENOUS FLUIDS AND SODIUM
The volumes of intravenous fluids administered to the patients during the first five days of hospitalization were recorded. These fluids included crystalloid fluids (0.9% saline solution; simple Ringer's solution, lactated Ringer's solution, or glucose solution), colloid fluids of any type, distilled water/drug diluting solution, medications, and blood or blood products. In addition, the total volumes of sodium infused intravenously on the first day and over the five days of hospitalization were calculated using the information on the products' leaflets.
PRIMARY ENDPOINT
The primary endpoint consisted in comparing the volume of intravenous fluids received by patients with or without caloric and/or protein deficits (according to the previously described limits). The volumes of sodium and sodium chloride administered to the two groups of patients were also compared.
STATISTICAL ANALYSIS
Continuous variables were initially analyzed using Levene's test to assess homogeneity, followed by the Kolmogorov-Smirnov test to determine normality. Student's t-test was used to compare continuous variables. Dichotomous variables were compared using the Fisher's exact test or the Chi-square test with Yates' correction. Data are expressed as mean ± standard deviation or as median and minimum-maximum. Statistical significance was set at 5% (p < 0.05). Statistical Package for the Social Sciences, version 17.0 (IBM Corp., Armonk, NY) was used to analyze the data.
RESULTS
Eighty-six patients hospitalized for a minimum of five days and on mechanical ventilation and on exclusively enteral therapy were prospectively studied. The patients' data are shown inTable 1. Mortality was 41.9% (n = 36) and median SAPS 3 score was 63 (interquartile range: 52.7-79).
Table I. Demographic, clinical, nutritional and biochemical data of the patients

SAPS 3: Simplifed Acute Physiology Score 3; CRP: C-reactive protein.Data expressed as median, minimum-maximum, or number of cases and percentage.
INFUSION OF INTRAVENOUS FLUIDS AND SODIUM
The patients received 2,969 (range: 920-5,958) ml of intravenous fluid/day in the first five days, which is equivalent to 41.6 (range: 17.0-88.2) m/kg/day. On the first day, 3,015 (range: 1,195-9,280) ml/day and 44.4 (range: 16-168) ml/kg/day of intravenous fluids were administered. During this period, the patients received 134 (range: 41-268) ml of sodium chloride/day and 10.7 (range: 3.3-21.4) g of sodium/day. Sixty-five patients (76.5%) received more than 30 ml of fluid/kg of body weight/day over the five days.
INFUSION OF ENTERAL NUTRITION
Enteral nutrition was initiated up to 48 h after admission in all patients. The mean percentage of enteral feeding infusion during the first five days was 67 ± 19.8% of the prescribed volume. Only 24.4% of the patients (n = 21) received ≥ 80% enteral feeding and 20.9% (n = 18) received < 50% enteral feeding in the first five days of hospitalization.
CALORIC AND PROTEIN DEFICITS
All patients exhibited caloric deficits, with the mean of 1,812 ± 850 calories over five days and 362 ± 170 kcal/day. Similarly, all patients had protein deficits (mean: 94.6 ± 45.9 g over five days and 18.9 ± 9.2 g/day). Twenty-three patients (27.0%) exhibited critical caloric deficits (≥ 480 kcal/day) and 34 (40%) exhibited critical protein deficits (≥ 20 g per day). Mortality was greater in patients with critical protein deficit (69% vs 41.1%; p = 0.01) but did not differ in patients with critical caloric deficit (58.7% vs 56.5%; p = 0.85) compared to patients without critical deficits.
Table II. Comparison of the extent of caloric deficits and the infusion of intravenous fluids, sodium chloride and sodium

Data expressed as mean ± standard deviation.
Table 2 shows the comparison between the volume of intravenous fluids, sodium chloride, and sodium infused to patients and the extent of their caloric deficits.Table 3 shows the comparison between these infused fluids and the extent of the patients' protein deficits. Critical protein deficits and critical caloric deficits were associated with a higher volume of infused fluids, sodium, and sodium chloride (p < 0.01 in all comparisons).
CLINICAL AND LABORATORY VARIABLES AND INTRAVENOUS FLUID
Table 4 and Table 5 show the comparison between the clinical, demographic, and laboratory variables and the extent of caloric and protein deficits, respectively. The level of serum albumin was significantly lower in patients with critical caloric deficits than in those without. There was no difference in the clinical and biochemical results between the patients with and those without critical protein deficits (Table 5).
Table IV. Comparison of calorie deficits and patients' data
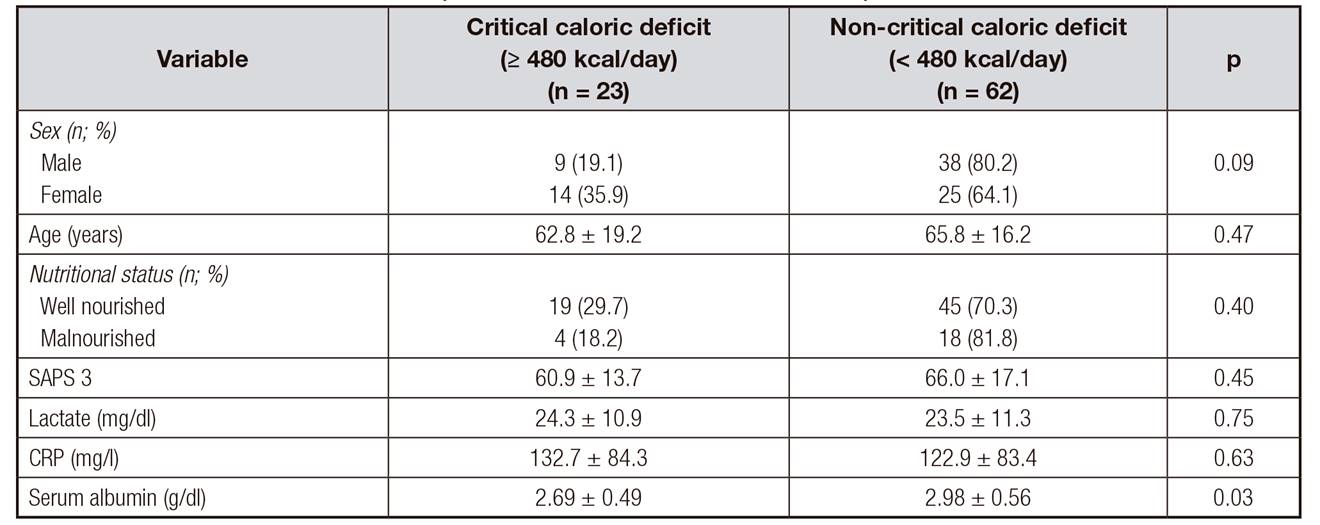
SAPS 3: Simplifed Acute Physiology Score 3; CRP: C-reactive protein. Data are expressed as mean ± standard deviation or number of cases and percentage.
DISCUSSION
The findings of this study indicate that excessive infusion of intravenous fluids and sodium is associated with caloric and protein deficits in critically ill patients receiving exclusive enteral nutrition. A positive fluid balance of fluids in critically ill patients may disturb gastrointestinal function by causing edema of the intestinal wall, gastroparesis, and ileus, all of which are associated with worse outcomes 1,18. Complications such as pulmonary edema, poor wound healing, and intestinal dysmotility can be reduced with fluid restriction 19,20.
In the present study, some patients received more than 80 ml of intravenous fluids/kg/day. This may have resulted in unrecorded gastrointestinal complications, which may have contributed to the caloric and protein deficits.
An increased mortality rate may be expected in patients who received aggressive fluid therapy 21. Since the publication of the early goal-directed therapy study 22, there have been significant changes in approaches to fluid resuscitation in case of critical illness. Several studies have indicated that the conservative use of fluids is more beneficial than liberal use 23,24-25. In most cases, approximately 30 ml/kg/day is sufficient for maintenance.
In the present study, an infusion of more than 30 ml/kg/day was recorded in up to 75% of the patients, which may have contributed to the lower infusion of enteral nutrition and increased caloric and protein deficits. This present study confirmed a previous study in our ICU 17 relating protein deficit in critically ill patients receiving enteral nutrition to greater mortality. Several studies have shown that the mean percentage of infused enteral nutrition is 50% to 70% of the prescribed volume 2,26,27-28. The excessive administration of fluids and sodium may have a role with that. In this context, caloric and protein deficits result in increased mechanical ventilation-free days, and increased length of hospital stay; these deficits represent an independent factor for death 18,29. Moreover, when caloric and protein targets are met, the result is reduced mortality rates 30,31. The adequate infusion of proteins reduces protein catabolism by 50%, improves nitrogen balance 32, and reduces days on mechanical ventilation and 33 mortality 34. In the present study, the mean percentage of infused enteral nutrition was approximately 70% of the prescribed volume. Patients improve when the percentage of infused enteral nutrition is higher than 80 35.
The results involving the total sodium volume administered to patients were very relevant. Sodium contributes to the occurrence of edema and anasarca. The infusion of saline solution is common but 0.9% sodium chloride is proven to be inadequate and "non-physiological" 36. Some patients receive up to ten times the adequate sodium volume 37 solely through the intravenous route, and sodium infused through enteral feeding needs to be added. According to a study, patients who receive more than 3 l of fluids and 154 mmol of sodium/day remain hospitalized for a longer period of time and exhibit delayed recovery of intestinal function 38.
The results of the present study show that the sodium volume may also have contributed to the observed caloric and protein deficits. Although the present study adds new data to the current literature on the topic, its limitations include the size and homogeneity of the sample. However, the overall results showed that overload of fluid infusion may impact the success of enteral nutrition infusion. These findings pave the way for further research on the topic. The current belief is that only gastrointestinal disorders and enteral nutrition formulation lead to enteral nutrition intolerance with subsequent caloric and protein deficits. To conclude, critical caloric and protein deficits are associated with a higher volume of fluids, sodium chloride, and sodium when these products are infused to critically ill patients on mechanical ventilation and receiving exclusively enteral nutrition.
















