INTRODUCTION
Fatty acids, the constituent elements of lipids, are organic components that contain carbon and hydrogen in their molecules. When short and medium-chain fatty acids (C4-C12) are not supplied by the diet, they are synthesized, mainly in the cytoplasm of hepatic and adipose tissue cells. The long-chain fatty acids (C12-C24) are supplied by the diet 1.
The process of fatty acid uptake and oxidation is of particular metabolic significance, both at rest and during light to moderate exercise. The fatty acids are the predominant source of substrate for adenosine triphosphate (ATP) resynthesis. During aerobic exercise, there is an increase in the release of fatty acids from the adipose tissue to accommodate the increased energy needs caused by exercise 2. As exercise progresses, long-chain fatty acids, provided by the blood or from hydrolysis of intramuscular triacylglycerols, are metabolized to generate energy. The supply of fatty acids from the hydrolysis of intramuscular triacylglycerols is limited and during exercise, the myocytes consume approximately 90% of the free fatty acids derived from blood plasma 3.
Studies have also suggested that physical exercise performed in an acute manner promotes changes in the fatty acid transport genes, preceding increases in RNAm expression 4. This allows optimal delivery of fatty acids to the transporters, and their greater metabolism, although the latter has not been tested yet 5.
The use of lipids is modulated by the availability of fatty acids in plasma and obesity may influence the increase in plasma AG levels 6. Glycolytic activity is changed in this population, and lipid metabolism becomes a preferential pathway. In obese subjects, the metabolic responses of fatty acid mobilization appear to be favored by aerobic activity; however, the responses are not conclusive yet 7.
Therefore, the aim of this study was to test the hypothesis that acute physical exercise would change the medium and long-chain fatty acids in the serum of individuals with increased body weight.
METHODS
STUDY DESIGN AND POPULATION
This randomized clinical trial, registered in clinical trial under protocol NCT03170973, was conducted with a population accessible to the School Clinic of the Adventist Faculty of Bahia, Brazil. Data collection was performed from September 2015 to May 2016.
All the women registered with the Physical Therapy Service of the Clinic School who had a body mass index (BMI) above 24.99 kg/m2 were invited to participate in the study. A total of 66 volunteers fulfilled the inclusion criteria, which were: age between 18 and 30 years, BMI > 24.99 kg/m2 and sedentarism. Sedentarism was determined based on the International Physical Activity Questionnaire - Long Version 8. Women who presented with cardiovascular and metabolic disease, hypothyroidism, renal parenchymal disease or diabetes mellitus, history of alcoholism or smoking, or use of hypolipemiant, corticosteroids, diuretics, beta-blockers, and contraceptive medications were excluded from the study.
The women were randomly divided into two groups: exercise group and control group, both with 33 volunteers.
EXERCISE GROUP
After 12 hours fasting, the volunteers were submitted to blood collection, in the antecubital vein, to measure the basal serum values of triglycerides, total and fractionated cholesterol, glycemia and insulin. The HOMA-IR and HOMA-β index values were calculated from the glycemia and insulin values, by means of the equation proposed by Mathews 9.
When 12 hours had elapsed after the first blood collection, the patients performed a physical exercise session on an ergometric treadmill. This was divided into three time intervals: warming up, conditioning and cooling down. The duration of the warming up period was seven minutes and of the cooling down, five minutes, and conditioning time corresponded to an energy expenditure of 250 kcal 10 with light intensity based on the Borg 11 rating of perceived exertion, that is, on the original scale: a value between 9 and 11. For better understanding of this scale, on the day before the exercise, the volunteers got familiar with the Borg concept in order to get used to providing adequate answers when they were asked about the intensity of the exercise. A cardiac frequency meter was used, which measured the energy expenditure based on the volunteer's body mass, sex and age.
After the physical exercise session, the volunteers were instructed to go home and keep to their habitual diet. When 24 hours had elapsed after the first blood collection, the volunteers returned to the laboratory after a 12-hour fasting, and once again had blood samples collected. The diet of the previous two days before the blood exam was evaluated by means of a 24 hours diet diary.
CONTROL GROUP
The women in the control group were submitted to the same data collection protocol as that of the experimental group, however, they did not do the exercise 12 hours after the first blood collection. They were instructed not to perform any physical exercise on the two days before blood collection, as shown in the flow diagram presented in Figure 1.
24-HOUR DIET DIARY
Once again, they were evaluated as regards their diet on the day before the exam by means of keeping a 24-hour food-intake diary. The 24-hour food intake diary was evaluated by means of an interview held at the time of blood collection, in which the volunteers informed what they had consumed on the day before the interview, in the three main meals and between these meals. The instrument was applied by the same examiner, and to facilitate responses, home measurements were used 12. Quantitative evaluation of the diet was performed by using the Avanutri Revolution software. For the purposes of analysis the consumption of the following were considered: micronutrients (vitamins and minerals), cholesterol, total saturated, monounsaturated and polyunsaturated fats and total dietary fiber, using the parameters of the Brazilian Society of Cardiology 13.
BLOOD COLLECTION AND FATTY ACID ANALYSIS
The volunteers were submitted to blood collection after fasting for 12 hours. Samples of 5 ml of blood were collected in tubes with EDTA, and after collection the samples were centrifuged at a speed of 3,000 rpm for ten minutes.
Fatty acids, which have 9C (azelaic and pelargonic) and 18C (oleic and elaidic) carbon molecules tested in this study are considered as medium- and long-chain fatty acids and would be able to be more easily metabolized in the mitochondria for energy production. In addition to this aspect, oleic acid is a fatty acid highly stimulated for consumption as it becomes interesting to understand how physical exercise interferes in this fatty acid as well as its geometric isomer (elaidic) and its by-products of metabolism (azelaic and pelargonic) 14.
The initial stage for fatty acid analysis was trans-esterification of the samples by means of successive stages. The first stage was extraction; then, the hydrolysis and esterification stages began. In the same way, the patterns at 99% purity of the fatty acids (pelargonic, azelaic, oleic and elaidic) were also trans-esterified in the different stages 15.
After trans-esterification of the patterns and samples, these were analyzed by gas chromatography 16 by means of the Thermo Scientific appliance, model Focus GC(tm), serial no.: 10902047. Methanol was used as solvent.
The maximum temperature used for conditioning the column was 230 °C, with readout time of 21.78 minutes and ramp of 30 °C/min 17.
Identification of the patterns proceeded with isolated readout of the samples in 21.78 minutes, to determine their respective retention times and area, followed by simultaneous readout, to construct a single pattern.
The retention times (t) and area (a) of the pelargonic, azelaic, elaidic and oleic acids were: 5.9 minutes and 537,138,819; 7.8 minutes and 113,433,890; 9.4 minutes and 1,274,291,989; and 12.9 minutes and 75,971,297, respectively.
ETHICAL ASPECTS
This study was submitted to the Research Ethics Committee of the Faculdade Adventista da Bahia and approved under protocol no. 34017514.5.0000.0042. Throughout the entire study, the guidelines on research with human beings of Resolution 466/2012 of the National Health Council were observed.
STATISTICAL ANALYSIS
The data were previously analyzed by the Shapiro-Wilk test, with regard to symmetry. For characterization of the following variables: BMI, age, HOMA-IR, HOMA-β, insulin, glycemia, TG, CT, HDL, LDL and TG/HDL, vitamins, minerals and fatty acids, the mean and standard deviation or median and interquartile interval were used, depending on the behavior of the variable. The level of significance was defined by the value of p < 0.05.
For comparison of the effects of exercise on the percentage of fatty acids (pelargonic, azelaic, elaidic and oleic) in the serum of obese women, inter- and intragroup comparisons were made by using the paired and non-paired Student's-t test in cases of symmetry. The data were analyzed by using the Statistical Package for the Social Sciences (SPSS) software program, version 24.0.
RESULTS
In Table 1 the authors observed that no differences were found when the clinical, anthropometric and lipid profile variables were compared. The insulin, insulin resistance and insulin sensitivity values were observed to be more elevated in the control group.
Table I. Clinical and anthropometric characteristics of the sample per control and exercise group, on the first day of blood collection (n = 66)

CG: control group; EG: experimental group. Mean ± standard deviation; Student's-t test. *p < 0.05.
*p < 0.05.
In Table 2 the intra-group analysis of the percentages of fatty acids contained in the serum of women with increased body weight may be observed. No differences between the control and exercise groups were found when the percentages of fatty acids before and afterwards were compared.
Table II. Intra-group analysis of the percentages of fatty acids contained in the serum of women with increased weight, before and after, in the exercise and control groups
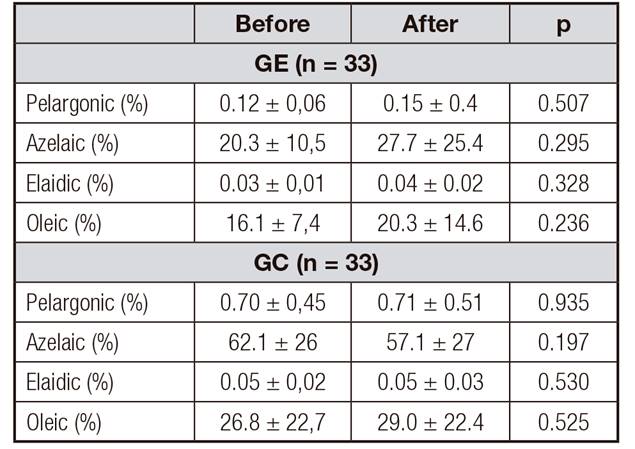
CG: control group; EG: exercise group. Mean ± standard deviation; paired Student's-t test.
When the intergroup analysis was performed of the differences (after/before) in the percentages of fatty acids contained in the serum of women with increased body weight, the authors observed that no differences were found between the control and exercise groups (Table 3).
DISCUSSION
In our studies, there was no evidence of differences in the percentages of plasma medium-chain fatty acids after low intensity physical activity. Studies have suggested that acute physical exercise promoted changes in the fatty acid transport genes, preceding increases in RNAm expression 4, and that the change in these transporters favored greater fatty acid metabolization 5. Moreover, it is possible that exercise changes the composition of the fatty acids in plasma 18. In our study, we observed that the medium- and long-chain fatty acids present in the plasma of individuals with increased weight did not undergo changes 12 hours after the practice of low intensity physical exercise.
The process of fatty acid uptake and oxidation is of significant importance, both at rest and during light to moderate exercises. The fatty acids are the predominant source of substrate for ATP resynthesis 2. Oxidative metabolism allows energy to be obtained from fatty acids in an intramitochondrial localization. Thus, to enable acyl-CoA to be used by it (oxidative metabolism), it is necessary to overcome the impermeability of the external and cytoplasmic membrane of the mitochondria to attain the acyl-CoA. The enzyme responsible for this transport is carnitine-CoA acyltransferase (carnitine O-palmitoyltransferase). This enzyme presents greater specificity for palmitoyl-CoA, however, it catalyzes transport of fatty acids with a carbonated chain length between C4 and C18. Fatty acid chains longer than these are more difficult to be transported. Once within the mitochondria, acyl-CoA may be used in the lipolytic metabolism of Lynen 12. The fatty acids, which have 9C (azelaic and pelargonic) and 18C carbon molecules (oleic and elaidic), tested in this study are considered as medium- and long-chain fatty acids, and would have conditions to be more easily metabolized in the mitochondria for energy production. These fatty acids would be expected to present reduced percentages after physical activity.
Different studies have been presented relating to the effect of physical exercise on lipids; however, the results are still controversial 7,19,20,21. Over the last few decades, growing evidence could be observed that acute physical exercise could have a beneficial effect on the lipid profile 22,23,24, and it has also been observed that its effect could last for up to 48 hours after the exercise session 25. It is worth pointing out that the majority of studies have evaluated the late effect of acute exercise on lipids after time intervals of 12, 24 and 48 h. The difficulty with analyses and interpretation of these studies lies in the use of different physical activity protocols established 26.
In studies that have evaluated the drop in triglycerides in the post-prandial period, the calorie expenditure with physical exercise was the determinant factor in the magnitude of the drop in triglycerides. These studies suggested that very low intensities might not present significant results, due to the fact that they do not attain an adequate calorie expenditure to cause a reduction in the triglycerides 27,28, contradicting studies that suggested the practice of low intensity activity for better effect on fats oxidation 7,29.
As observed in table I, the control group presented a higher insulin index when compared to the exercise group. Since the increase in insulin could limit the mobilization of fatty acids 30, greater fatty acid mobilization was to be expected in the exercise group. However, in spite of the lower insulin values in the exercise group, this aspect did not favor greater fatty acid mobilization, because no differences were observed in the pelargonic, azelaic, oleic and elaidic values between the control and exercise groups.
Among the limitations observed in this study, the following are included: a single time interval of observation of the responses related to fatty acids 12 hours after exercise, and absence of caloric expenditure above 250 kcal for different comparisons. With reference to physical activity, factors such as calorie expenditure and time of observation may have a strong influence on the metabolic responses 31. This study presented the responses to a calorie expenditure and specific time, and for these variables, no changes were observed in the different fatty acid values, which cannot be affirmed for time intervals longer than 12 hours and calorie expenditures different from 250 kcal. Different physical exercise protocols must be investigated to contribute further elucidation about the effects of physical activity on the medium- and long-chain fatty acids.
















