INTRODUCTION
Obesity has become a worldwide epidemic and it has been projected that its prevalence will grow by 40% over the next decade 1. This increasing prevalence is related to the increased risk of diabetes, cardiovascular diseases and also chronic kidney disease 2.
Some of the deleterious renal consequences of obesity may be mediated by other comorbidities such as diabetes mellitus or hypertension, but there are also adiposity effects that may directly affect the kidneys, induced by endocrine activity of adipose tissue through the production of adiponectin, leptin and resistin 3. These include the development of inflammation, oxidative stress, abnormal lipid metabolism, activation of the renin-angiotensin-aldosterone system and increased insulin production and insulin resistance 4.
Recently, obesity has been identified as a nutritional disorder that is also common in patients with CKD 5, and the literature has pointed out that a high body mass index (BMI) is one of the strongest risk factors for the development of chronic kidney disease 2, once it seems to be associated with the reduction of the estimated glomerular filtration rate with faster loss over time and with the incidence of end stage renal disease 6.
Definitions of obesity are most often based on BMI. According to the World Health Organization (WHO), values of BMI equal to or greater than 25.0 kg/m² and 30.0 kg/m² are used to identify overweight and obesity, respectively 7. Although it is easy to obtain BMI, this is an imprecise estimate of the distribution of body fat. Besides, it does not differentiate lean mass and fat mass. Thus, body fat may differ within the same BMI and there is evidence that patients with CKD have a higher percentage of fat mass when compared to control subjects 8,9.
The estimation of the %BF has a better validity for the identification of excess body fat 10. Thus, other laboratory techniques have been developed and improved to estimate %BF as dual-energy X-ray absorptiometry (DEXA) and air displacement plethysmography (ADP).
DEXA is more accurate than BMI to measure body fat, both in young individuals and in the elderly. Besides, it is sensitive to small changes in body composition 11. It is considered as a tool of choice by the recommendations of European and American societies of nutrition to evaluate the body composition of renal patients and has been used as a reference in comparative studies of body composition in hemodialysis patients 12.
ADP has been adopted in clinical practice and is considered as a gold standard for assessing body composition 13. Some studies have been conducted to evaluate its efficacy both in the different clinical conditions and in comparison with other available methodologies, being considered as reliable 14,15.
The ADP and DEXA, used to evaluate the body composition of patients with CKD, have shown to be sensitive and efficient; however, the PDA presents less limitations, since the use of DEXA is not indicated in obese individuals, with total body weight over 150 kg and diameter of the waist circumference (WC) that exceeds the width of the equipment 16.
Considering the increase in the prevalence of obesity in patients with CKD, and the fact that the cut-off points of anthropometric indexes universally used for its diagnosis in the general population may not present the same performance in these individuals, this study aimed to determine the sensitivity and specificity of BMI for obesity detection in renal patients on conservative treatment through comparison with the adiposity indexes obtained by DEXA and ADP, as well as to identify the most accurate cut-off points for the detection of body fat in these individuals.
METHODS
A cross-sectional study was carried out in patients with non-dialysis chronic kidney disease attended at the Center for the Prevention of Renal Disease of the University Hospital of the Federal University of Maranhão (HUUFMA). This study is part of a larger project entitled "Inflammation and cardiovascular risk in patients with non-dialysis chronic kidney disease, São Luís - MA", which was approved by the Research Ethics Committee of the Federal University of Maranhão (Permission nº 2.015.866).
Adults and elderly men and women with a diagnosis of chronic kidney disease undergoing conservative treatment were invited to participate in the study. Pregnant women, patients with amputations, patients with only one kidney, hospitalization in the previous month, patients with a history of dialysis, hepatic insufficiency, chronic consumptive diseases such as cancer, severe heart failure, acquired immunodeficiency syndrome and infectious diseases were not included.
The screening of individuals eligible to participate in the study was performed by a doctor, during outpatient care, with referral to the nutrition office. During the nutrition consultation individuals were informed about the study and invited to participate. Those who agreed to participate signed the informed consent form, being preceded by joint reading (nutritionist and patient).
This study was carried out in two stages. In the first stage, an interview was carried out to collect data such as demographic, socioeconomic, lifestyle data and clinical data, such as the presence of arterial hypertension, diabetes mellitus and CKD stage. Age was categorized as being less than 40 years, between 40 years and 59 years, and equal to or greater than 60 years. The schooling was evaluated in years of school attendance and classified in < 9 years and ≥ 9 years. Skin color was self-reported 17 as white, brown, black and others. Family income was categorized as monthly minimum wages (MMW): ≤ 1 MMW; > 1 and ≤ 2 MMW; > 2 and ≤ 4 MMW and > 4 MMW.
Lifestyle was assessed through data such as smoking, alcohol consumption and physical activity. Smokers were those who reported cigarette use during the study period, regardless of the amount. Similarly, those who reported alcohol consumption were considered to be alcohol consumers.
In order to assess the level of physical activity, the International Physical Activity Questionnaire (IPAQ - 8.0) was used in its short version, proposed by the WHO 7. Based on the data provided by the IPAQ, the time spent by each individual on physical activities (PA) of different level was calculated: high (vigorous PA), moderate (moderate PA), light (light PA), the sum of time spent in the three levels (total PA) and activities related to sedentary behaviors (rest). All values were expressed in minutes/day. Patients with a score > 150 minutes per week were considered as active, those below 150 minutes of activities per week were considered as irregularly active, and those who did not perform any physical activity for at least ten continuous minutes during the week were considered to be sedentary 18.
In the second stage, a nutritional assessment was carried out. Initially, anthropometric measures (weight, height) were taken. Body composition tests were then performed: computerized densitometry by DEXA and ADP.
Body weight was measured using a calibrated scale (Filizola(r), Brazil) with a maximum capacity of 150 kg and subdivisions per 100 g. Height was measured with the aid of a portable stadiometer (Alturexata(r), Brazil) with a scale of 0 to 220 cm and a precision of 0.1 cm.
The weight adequacy for height was evaluated according to BMI, obtained by the ratio between body weight and height squared, and adopted two classifications: the one proposed by the WHO (7) for adults and the one proposed by Lipschitz 19 for the elderly.
The DEXA test is a high-tech imaging procedure that allows the quantification of fat and muscle, as well as the bone mineral content and deeper bone structures of the body. The test was performed with the individual lying supine on a table using the equipment (Lunar DPX NT-GE healthcare(r), Brazil) where the source and detector scanned the body at a relatively slow rate of 1 cm/s. To allow a reconstruction of the underlying tissues image, allowing quantification of bone mineral content, total fat mass and fat-free body mass, a software (ENCORE-GE healthcare(r), USA) was used. For this study, data regarding the percentage of total fat (%TF) were used.
ADP was performed observing the criteria described in the equipment manual and by Higgins et al. (2001) and Fields et al. (2004). To do this, the device (BOD POD-COSMED(r), Italy) was initially calibrated; then, the subject was weighed in the equipment scale and, finally, the measurement of the volume occupied by the patient was done, observing the principle of Boyle.
During the whole test, the subject remained seated inside the equipment and did not wear any metallic objects such as earrings and rings while inside it. The subject wore a specific clothing provided by the researchers and was asked to perform three respiratory incursions. If these incursions were performed above an acceptable standard, the equipment software itself rejected the values obtained and therefore a new assessment was required until it was considered as adequate.
Thus, variations between pressure and volume were measured to determine body density. Once this data were obtained, the body composition was measured based on the Siri equation 20. Using the equipment own software, data referring to the percentage of total fat (%TF) were used.
The BF percentages obtained by DEXA and ADP were classified according to Jackson and Pollock (1980), who considered as "high" BF values between 23 and 28% for men and between 31 and 35% for women, according to the age range.
The data were expressed as mean and standard deviation (mean ± SD) for the continuous variables and frequency and percentages for the categorical variables. The normality of the variables was evaluated by the Shapiro-Wilk test. Categorical variables were analyzed using the Chi-square test. The Pearson correlation coefficient was used to test the associations between the variables. The Student's t-test was used to compare the sexes in the comparison between means of obesity and overweight.
To evaluate the diagnostic performance of BMI and WC in the assessment of excess body fat, the receiver-operator curve (ROC) analysis was applied. Diagnostic accuracy refers to the ability of BMI to discriminate patients with excess of those without excess body fat. In order to do that, the area under the curve (AUC) and the confidence intervals in the ROC analyses were determined. Then, sensitivity and specificity between the anthropometric indicators and the %BF obtained by DEXA and ADP were calculated. The cut-off points were those that presented a more adequate balance between sensitivity and specificity for the measures analyzed.
A significance level of 5% was adopted and the analyses were performed using the statistical package STATA version 14.0(r).
RESULTS
A total of 78 chronic renal patients on non-dialysis treatment were evaluated. The mean age was 54.4 ± 13.9 years and there was a predominance of individuals over 60 (43.6%), of the self-declared brown color (66.7%), with less than nine years of schooling (52.0%) and with family income of up to two monthly minimum wages (65.4%). The most prevalent disease was hypertension (93.6%) and 34.6% of patients were in stages 3A and 3B of CKD (Table 1). Regarding gender, women had less schooling (66.7% vs. 28.9%, p = 0.001).
Table I Sociodemographic and clinical characteristics of renal patients on conservative treatment
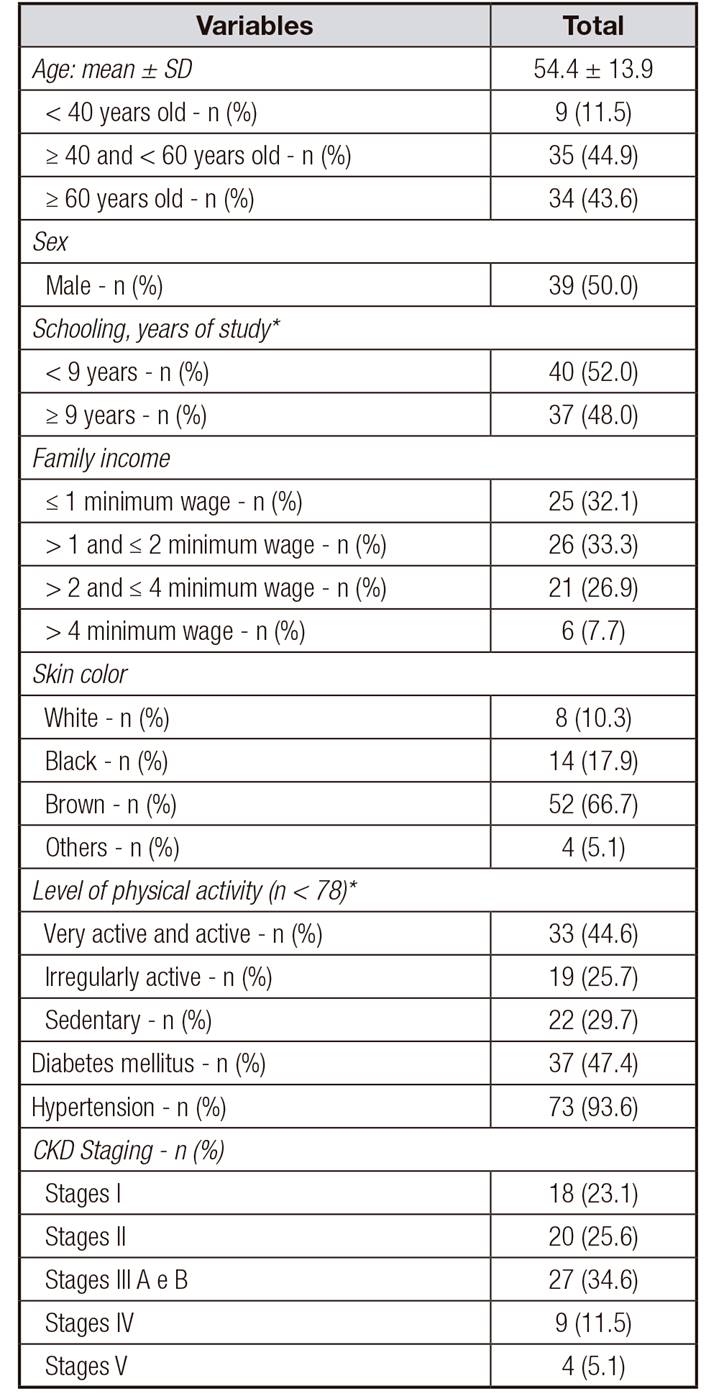
*n < 78. CKD: chronic kidney disease.
Nutritional characteristics of the participants are shown in Table 2. According to BMI, there was a higher prevalence of overweight/obese individuals (55.2%) and high %BF, according to DEXA (69.2%) and ADP (53.8%). When splitting up the sample by sex, the women had higher mean values of %BF according to DEXA (37.0 ± 8.4 vs. 31.5 ± 9.1, p = 0.007) and ADP (33.7 ± 9, 5 vs. 27.9 ± 10.3, p = 0.012) (Table 2).
Table II Nutritional characteristics of renal patients on conservative treatment

BMI: body mass index; %BF: percentage of body fat; DEXA: dual-energy X-ray absorptiometry; ADP: air displacement plethysmography.
It was observed that the BMI correlated with the %BF obtained by DEXA and ADP in both sexes (p < 0.01) (Table 3). According to the values of the area under the ROC curve, the BMI showed a statistically significant predictive capacity to identify individuals with %BF in both sexes and through the DEXA and ADP, since the lower limit of the 95% CI of the area under the curve (AUC) was > 0.50 (Table 4).
Table III Pearson correlation between BMI and BMI with the percentage of total fat obtained by DEXA and ADP

BMI: body mass index; %BF: percentage of body fat; DEXA: dual-energy X-ray absorptiometry; ADP: air displacement plethysmography; Coef: Pearson correlation coefficient.
Table IV Area under BMI curve with percentage of total fat obtained by DEXA and ADP

95% CI = 95% confidence interval; AUC: area under curve; DEXA: dual-energy X-ray absorptiometry; ADP: air displacement plethysmography; BMI: body mass index.
Sensitivity and specificity values for BMI cut-off points compared to %BF, obtained by reference methods for body composition assessment (DEXA and PDA), are shown in Table 5. To detect high %BF, a BMI of 25 kg/m2 had better sensitivity and specificity values for DEXA (73.3% and 66.7%, respectively) and ADP (77.3% and 52.9%, respectively)) in males, and for DEXA (79.9% and 46.7%, respectively) in females. However, for the ADP a BMI of 26 kg/m2 would be a more accurate cut-off level (70.0% and 73.7%, respectively).
DISCUSSION
The predominance of patients with excess body fat, evaluated by both BMI and %BF, was high. There was a statistically significant correlation between BMI and %BF obtained by DEXA and PDA. However, the cut-off points of these indexes, traditionally used to detect obesity in the general population, were not adequate for these patients.
The predominance of overweight and obesity worldwide has increased substantially 21 and it is an important risk factor for the development and progression of CKD, regardless of the concomitant occurrence of other comorbidities such as HT and DM 22.
Several studies have demonstrated an association between higher BMI and the presence of proteinuria in individuals without renal disease 23,24,25. Other studies have shown an association between obesity measures and the development/progression of CKD 26,27, with a more rapid decrease of eGFR over time and the incidence of CKD in its terminal phase 28.
On the other hand, some researchers suggest that obesity is favorable for the survival of patients with CKD 29,30. However, it is possible that the apparently protective effect of a high BMI is the result of the imperfection of BMI as a measure of obesity, since it is not able to distinguish the fat mass from the fat-free mass 31.
Considering that the concept of obesity is defined as excess body fat 7, it is known that in the absence of a simple technique to determine this measure in the population, BMI ends up being widely used as a tool to evaluate this excess fat.
In this study, according to the cut-off point for BMI (≥ 30 kg/m²) recommended by the WHO for the general population, the predominance of obesity was 9%, much lower than that observed with DEXA (69.2%) and ADP (53.8%). Thus, it suggests that this cut-off point underestimates the identification of obesity (excess body fat) in these patients.
The sensitivity and specificity indexes of BMI in the diagnosis of obesity in renal patients are one of the main points of academic discussion. According to some authors, this parameter of diagnosis of obesity should not be used alone, especially in the population with CKD, once it does not distinguish the type of tissue 32 and the body changes already discussed 33.
Analyzing the cut-off points of the BMI with the gold standard methods for diagnosing obesity in the study group, it was observed that BMI = 25 kg/m2 showed better sensitivity and specificity values for the detection of high BF for men. Therefore, it can be used in the screening of patients with CKD, since a high sensitivity is essential. On the other hand, a BMI of 30 kg/m2, recommended by the WHO, has low sensitivity but high specificity, which means a large number of false negatives, with a higher predominance of people being diagnosed as eutrophic despite being actually obese.
A likely explanation for the underestimation of %BF obtained by the BMI is that this index is not able to consider the loss of lean mass concomitant to fat gain in individuals with CKD, a condition characterized as sarcopenia 34). In these patients, loss of muscle mass is manifested in isolation or in conjunction with an increase in fat mass 33.
The results found in this study corroborate those of other authors that suggest reviews to the BMI cut-off points. Romero-Corral et al. (2008) 35 found BMI values for obesity classification for North American individuals 25.8 kg/m² for man and 25.5 kg/m² for women. In a study of 77 individuals (63 males and 14 females) with spinal cord injury, Laughton (2009) 36 considered BMI ≥ 22 kg/m² as a high risk for developing chronic diseases related to obesity.
In 2004, a meta-analysis of the BMI values for the Asian population was published by the WHO given the high prevalence of type 2 diabetes and cardiovascular diseases in people with BMI below the suggested cut-off point for pre-obesity (25 kg/m2). The WHO then agreed that the cut-off points suggested for the world population in 1998 did not fit this ethnicity and suggested lower cut-offs for the population: above 23 kg/m2 for pre-obesity, 27.5 kg/m2 as obesity grade I, 32.5 kg/m2 as obesity grade II and grade III as 37.5 kg/m2. Moreover, it was also proposed that each country could make its own decisions on definitions of population risk 37.
For women, a better sensitivity value was observed for the BMI = 25 kg/m2 cut-off point compared to the %BF obtained by DEXA, while the BMI of 26 kg/m² would be more accurate for the detection of %BF obtained by ADP, since they presented better values of sensitivity and specificity.
The cut-off point for the diagnosis of obesity by BMI (≥ 30 kg/m²), proposed by the WHO (1998), showed a greater sensitivity (85%) using the ADP method as a reference; in other words, a higher prevalence of misdiagnoses of obesity. Guimarães et al. (2017) 38 suggest the use of BMI = 25 kg/m2 for the diagnosis of obesity, with sensitivity values of 82% and specificity of 58% for women with rheumatoid arthritis, and a mean age of 55.2 years. Like the studied population, this group of women presented loss of muscle mass and a muscular fat infiltration due to the inflammatory process 33,34. This may explain the higher %BF despite a BMI within normality. Thus, the concept of obesity in patients with non-dialysis CKD should be managed carefully.
This study had as limitation the sample size and the heterogeneity of the sample in terms of age, race and stage of CKD. As a positive aspect, it included individuals of both sexes, was carried out in a population with few studies on this subject and used gold standard methods to identify %BF. It should be noted that a larger number of patients would be necessary to determine the most appropriate cut-off point for each age, race and stage of chronic kidney disease (CKD).
The findings of this study will contribute to the early and more accurate diagnosis of obesity, promoting more adequate interventions that prevent obesity-related complications.
It is important to highlight that the cut-off points for the BMI suggested were estimated by determining the body fat of the renal patients analyzed, and for this reason they do not refer to the low weight of fat free mass but to cut-off points where the amount of body fat, estimated through the DEXA and ADP, were considered as "high" values for men and women, according to age group.
Considering the DEXA and ADP methods as gold standard, cut-off points conventionally used for anthropometric indexes in the determination of obesity for the general population were not adequate for patients diagnosed with CKD in conservative treatment.
Besides, this study suggests that the BMI value = 25 kg/m2 is the most appropriate cut-off point for the diagnosis of obesity in patients with chronic kidney disease in the early stages of the disease, in order to promote early interventions and to prevent complications related to obesity. The importance of this study can be attributed to the fact that studies of this nature are incipient in these subjects. In addition to contributing to better prescriptions and, consequently, delayed progression of CKD, the high cost of renal replacement therapy (RRT) is one of the reasons justifying the importance of early CKD prevention and treatment.















