INTRODUCTION
Bariatric surgery is actually the best option to obtain a significant and maintained weight loss in patients with morbid obesity. However, nutritional deficiencies are often consequences of the bariatric approach, mainly in malabsorptive techniques 1. Thus, nutritional supplements are recommended to handle these deficiencies. Cases of malnutrition that do not respond to intensive (oral or parenteral) treatment are uncommon 2,3. These cases should be closely followed because they can hide other pathological conditions.
As a general rule, almost all mixed or malabsorptive bariatric techniques exclude a fixed portion of the small intestine, regardless of the total length of the gut 4. This occurs in techniques as widespread as Roux-en-Y gastric bypass (RYGBP) or the biliopancreatic diversion (BPD). Even single anastomosis techniques such as the minigastric bypass (MGB) exclude 200 cm of proximal bowel, or the single anastomosis duodenoileal bypass (SADI), which excludes 300 cm of distal bowel (counting from the iliocecal valve) 5. In all of these cases, surgeons do not know the total length of gut. Therefore, some patients show inadequate weight loss or signs of severe malnutrition that require revision surgery. In the one anastomosis gastric bypass (OAGB) technique (BAGUA in Spanish), the malabsorptive limb is customized to each patient 6. In this sense, the best attitude to perform a surgery would be: a) to determine the total bowel length, in order to adapt the degree of malabsorption to each patient, allowing effective weight loss with the minimal deficits; and b) to establish long-term follow-up to prevent possible deficits and quickly establish the appropriate treatment.
CASE REPORT
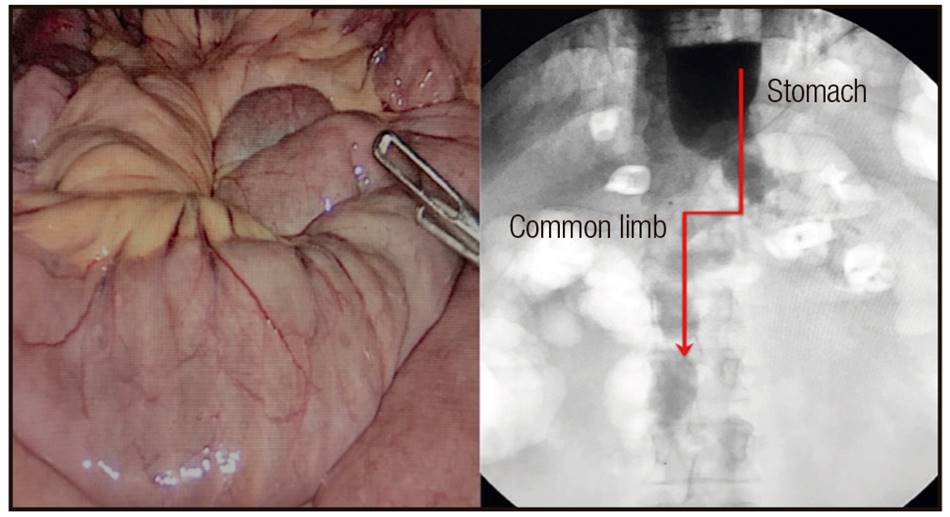
A 47-year-old man, with BMI 48 kg/m2, hypertension, obstructive sleep apnea-hypopnea syndrome, liver steatosis and arthropathies, underwent an OAGB in December 2014. Total bowel length was 490 cm, leaving 320 cm of the biliary limb and 170 cm of the common limb (Fig. 1). One year after surgery, the patient was asymptomatic, with BMI 26.6 kg/m2, and he was taking the prescribed oral multivitamin supplementation. In 2016, he complained of fever and cough and was admitted to hospital with severe sepsis and respiratory distress. Pneumonic infiltrates were observed in the X-plain rays. Cultures were negative. Prophylactic treatment with ceftriaxone, levofloxacin and oseltamivir was initiated and admission to the Intensive Care Unit (ICU) was required. The patient recovered from the respiratory infectious episode, but later on he began with nutritional deterioration, presenting 6-7 diarrhea/steatorrhea events per-day and requiring three hospital readmissions during the subsequent months. Laboratory data revealed hypoalbuminemia (2.4 g/dl) and hypoproteinemia (4.7 g/dl). During hospitalization, the patient was treated with 20% human albumin (50 ml/8 h iv) and received total parenteral high-protein (60% of the daily energy requirement [DER]) low-fat nutrition. Additionally, oral low-fat diet without lactose and saccharose (40% DER) was administered. After 31 days of hospitalization, the patient was discharged with improvements in clinical and laboratory parameters. However, the protein loss recurred. Distal ileoscopy revealed atrophy of the villi. Afterwards, the polymerase chain reaction (PCR) in blood and feces revealed the presence of Tropheryma whipplei. Then, diagnosis of Whipple's disease (WD) was established.

Figure 1 One anastomosis gastric bypass (OAGB) technique. The lengths of the limbs are customized to each patient. In this case 320 cm (biliary limb) x 170 cm (common limb).
Treatment with doxycycline (200 mg/day orally) and hydroxychloroquine (600 mg/day orally) was prescribed for 12 months.
After antibiotic treatment, the q-PCR tests were negative, but total recovery of protein deficiency was not achieved, despite supplementation with hyperproteic supplements (Atempero(r), VEGENAT, Spain). The clinical multidisciplinary committee evaluated the case and, suspecting intestinal damage after WD infection, revision surgery was decided in order to increase the absorptive common limb. Surgical findings showed a generalized thin-walled, flabby and dilated bowel. The intestinal transit was reconstructed with 250 cm of the biliary limb and 240 cm of the common one. The patient recovered uneventfully after this surgery (Fig. 2). Three months after surgery the patient was asymptomatic, with a BMI of 23.7 kg/m2 and correct blood parameters. The patient's quality of life improved and in the medical controls we reinforce the need to adopt healthy habits and to perform regular physical activity in order to avoid regaining weight. Our health care team has systematically offered advice regarding eating habits compatible with a balanced healthy diet and the intake of usual vitamin supplements was monitored.
DISCUSSION
Bariatric surgery has probed to have excellent results for the resolution of obesity and its comorbidities 1. However, the malabsorptive procedures might cause nutritional deficiencies, thus, long-term follow-up is mandatory 3.

Figure 3 Anatomical schema of the parts where nutrients are absorbed. In bariatric surgery, any component absorbed in the jejunum-ileum part is susceptible to show deficits.
Carbajo et al. advocate customizing the bowel limbs according to the total bowel length, not only based on the initial BMI of the patient, but also in relation to their comorbidities, age and gender 6. Few groups around the world perform bowel measurements, since it is a complex maneuver that involves technical risks, including unnoticed injuries to the intestinal. After OAGB from this group, some patients developed nutrient deficits (usually within the first 2-3 postoperative years) during the intestinal adaptation. Most of them were controlled and treated on an ambulatory basis and recovered with dietary supplements 6. A total of 14 patients of this series (1.2%) required further treatment due to hypoalbuminemia; all received high-protein enteral supplements and pancreatic enzymes (10,000-25,000 IU; Kreon(r), Abbott, Germany) with each meal during six months. Only two patients were readmitted and managed with iv albumin, and so far in the series of more than 15 years of monitoring, conversion of the technique to normal anatomy has not been required 7.
The point to be discussed here is that in patients with bariatric surgery, any episodes of diarrhea and malnutrition are usually attributed to surgery 8. This is true if these episodes are observed in cases of dietary transgressions or patients undergoing periods of biological stress 9. But outside these circumstances, any cases of hypoproteinemia that do not respond to intensive treatment should be evaluated cautiously 10. When cases of persistent hypoproteinemia are observed despite oral or parenteral rescue, it is crucial to make a good differential diagnosis, since re-operation not always will solve the problem 11. In this sense, the clue that led to the differential diagnosis in this clinical case was nutritional deterioration, which began just after the respiratory syndrome.
Whipple's disease (WD) is a rare, systemic infectious disease caused by the bacterium Tropheryma whipplei. Although rare, it may mimic a wide spectrum of clinical disorders and may have a fatal outcome. It often causes diarrhea, steatorrhea and weight loss. Protein-losing enteropathy can also occur 12. Other atypical presentations include endocarditis, pneumonia and/or central nervous system disorders, always with negative cultures in conventional media 13. In endoscopy, the mucosa appears pale yellow, dilated, thickened and rigid, with a fibrinous exudate on the surface. The mesenteric lymph nodes are enlarged with ecstatic lymphatic vessels. Gut biopsies show, microscopically, there is villous atrophy and distension of villi by infiltrates of foamy macrophages, which replace the lamina propria and may extend into the muscularis mucosa or submucosa. The macrophages have coarse granules in their cytoplasm, comprised of clumps of degradation products, which stain a brilliant magenta color with PAS 14.
Treatment guidelines suggest starting with an initial two-week antibiotic treatment followed by long-term maintenance therapy (1-2 years) to prevent relapses. Currently, doxycycline in combination with hydroxychloroquine during 12-18 months is the elective treatment regimen 12,14. The genetic predisposition of the host, rather than the genotype of the bacterium, influences the predisposition and severity of the infection. The evolution of bowel wall damage has not been clearly described in the literature. After the antibiotic treatment, the presence of the bacteria in the biopsies is eliminated and the mucosal villi are recovered; however, sequelae of intestinal lipodystrophy have not been described yet. Long-term follow-up for the rest of their lives is mandatory on patients who have suffered this infection 13,14,15.
The potential to develop serious complications exists if patients are not adequately followed after any bariatric surgery technique. However, when the patient is correctly managed, persistence of malnutrition should guide other possible causes. WD is a rare cause of malnutrition and the delayed diagnosis can be fatal. If the revision surgery had been done in this case, as the only justification for malnutrition, it is likely that another damage caused by the untreated infection might have happened.