INTRODUCTION
Small for gestational age (SGA) is defined as a birth weight below the tenth percentile birth weight for an infant of a specific gestational age and sex 1. SGA has associated with increased infant and child morbidity and mortality 2,3. A growing body of evidence has linked SGA to the risk of developing chronic diseases such as metabolic disease in adulthood 4. SGA prevalence changes depending on birth weight standards and population 5. Besides, although the prevalence of SGA infants is high among the low and middle-income countries 6, the SGA is also increasing in developed countries such as Europe, reaching figures around 10.5% 7. Risk factors for SGA include maternal age, body mass index (BMI), chronic diseases, socioeconomic status and unhealthy lifestyles 8. Only a number of these factors can be modified. Among the modifiable risk factors, maternal nutrition plays a crucial role, influencing fetal growth and birth outcomes 9.
Nutritional requirements for many nutrients increase during pregnancy in order to support fetal growth 10. This may produce several maternal deficiencies of essential nutrients such as protein, vitamin B12, iron, iodine, folate and thiamine 11,12. An inadequate intake of all of these nutrients has been linked to maternal morbidities, neurodevelopmental disease in babies and a higher risk of SGA in the newborn 13,14,15,16. In fact, inadequate intake of protein-energy is related to fetal growth failure 17. A balanced energy protein supplementation has been associated with a risk reduction of SGA around 32% and increasing birth weight by 73 g 18. Furthermore, anemia due to low nutritional iron intake is associated with the birth of small babies, affecting an average of 56% of pregnant women in developing countries and above 18% in developed countries 19. As a result, recommendations on supplementation or fortification food during pregnancy for these nutrients have been formulated. For this reason, pregnancy dietary counselling recommend the supplementation of these nutrients 20.
Among the dietary sources, meat and meat products provide the recommended daily amounts of these nutrients, especially of protein and heme-iron 21. Thus, meat intake should be related to a lower frequency of some nutritional deficiencies, such as iron, protein, iodine vitamin B12 and other vitamins, and by this pathway could be possible to reduce the risk of low birth weight (LBW) and SGA newborn 22.
A healthy diet during pregnancy should include moderate amounts of meat and meat products 23. Nowadays, maternal dietary advice is based on the reduction of the consumption of this food group in order to reduce the risk of exposure to potentially harmful food pathogens, such as toxoplasmosis, found in raw or undercooked meat 24. However, the occurrence of this infection among pregnant women in developed countries is unusual 25.
Few studies have analyzed the role of meat and meat products on the risk of SGA newborn, showing inconsistent results. Probably by the differences in nutritional status of the study population and the methods used to evaluate the dietary intake. Consequently, some of them have shown risk associations on SGA delivery 26,27 meanwhile other authors have showed protective associations 28,29.
The aim of this study is to analyze the association between maternal dietary intake of meat and meat products during pregnancy and the risk of having a SGA newborn in Spanish population.
MATERIALS AND METHODS
The study population includes women attended to at five hospitals of Eastern Andalusia (Spain): Hospital Universitario de Jaén (HUJ), Hospital de Úbeda (UB), hospitales universitarios de Granada (two centers) (HUG) and Hospital de Poniente (HP), serving 1.8 million people. Case and control groups were collected from May 15th, 2012, through July 15th, 2015. The Ethics Committees of the hospitals authorized this study. Informed consent was sought from every eligible woman.
The appropriate sample size was estimated based on the results of a similar study 27. To detect a significant (p < 0.05) OR of 0.6 between extreme quintiles with a statistical power of 80%, it was estimated that 447 pairs of cases and controls were required.
CASES
SGA was defined as having a birth weight smaller than the tenth percentile for the infant's gestational age when compared with that expected for the same gestational age and sex, according to tables previously developed for the Spanish population 30, without congenital malformations during the study period and residence in the referral area of the hospital. Nineteen women rejected participation. A total of 533 cases were selected: 79 (HUJ), 369 (HUG), 46 (UB) and 39 (HP).
CONTROLS
A match pair by age at delivery (± 2 years) was selected within the next week after including a case at the same hospital. Eligible women were those having a non SGA newborn with the same inclusion criteria for cases (residence in the referral area of the hospital and no malformations). Sixty-five women declined participation.
DATA COLLECTION
Information was obtained in both groups on general sociodemographic habits and personal characteristics, including age at pregnancy, ethnicity, education level, marital status, monthly income, socioeconomic class, occupation, adherence to Mediterranean diet (MedDiet adherence) and body mass index (BMI). BMI was calculated as weight in kilograms/height in m2. Weight and height were obtained from medical records of women if possible, or self-reported if not. Social class was coded in five levels ranging from I (the highest) to V (the lowest level) according to the classification of the Spanish Society of Epidemiology 31, which is near to that of the other authors reported in the Black report 32. To measure the MedDiet adherence, the index proposed by Trichopoulou 33 was used, which includes the following components: intake of vegetables, legumes, fruits, cereals, fish, meat and meat products, milk and milk products, with scores ranging from 0 to 9, with higher scores indicating greater adherence. Obstetric history comprised parity, antecedents of abortions, previous adverse perinatal outcomes and morbidities during pregnancy (preeclampsia, diabetes, infections and other obstetric conditions). Birth weight was measured as weight in grams in the delivery room. Toxic habits (smoking during pregnancy and alcohol consumption during and before pregnancy) were assessed with a structured questionnaire, which included the number and type of drinks during a week, specifying the day at the week and holidays. Drugs and medication including prescribed medications and over-the-counter drugs. Finally, the prenatal care (number of visits and date of first visit) was also considered. Prenatal care was measured by using the Kessner index. This index takes into account timing of entry in prenatal care, number of prenatal visits and gestational age at delivery 34.
DIETARY ASSESSMENT
Information on dietary habits in both groups was collected. Trained midwives gave a food frequency questionnaire (FFQ) to women after birth, previously validated in Spain, with open questions about the use of dietary supplements 35,36. All participants were asked to complete and return the questionnaire, being a criterion for the participation of the study. FFQ included a 137-item and allowed the classification into eleven food groups (dairy products, meats and meat products, fish and seafood, vegetables, fruits, cereals, potatoes, fats, nuts, precooked food, sauces and beverages). The questionnaire included nine response options (never or almost never, 1-3 times a month, once a week, 2-4 times a week, 5-6 times a week, once a day, 2-3 times a week day, 4-6 times a day and more than six times a day). For each food item, we estimated the average amount of food consumed (grams) multiplied by the intake frequency, the average total energy intake and the average intake of macro and micronutrients. A dietitian updated the nutrient data using the information recorded in the Spanish tables of food composition 37,38).
Meat and meat products included in the FFQ were: meat group, including chicken with/without skin, beef, pork, lamb and rabbit; and meat products group, including liver (beef, pork and chicken), other entrails (brain, heart and sweetbread), cured ham, cocked ham, processed meats (sausages, black pudding, etc.), pâté, hamburger and bacon.
Meat and meat products sizes were pointed out in the FFQ and the midwives gave examples of portion sizes according to groups predefined as follows: meat group (100-150 g), liver and other entrails (100-150 g), ham cured/cooked (one slice or 30 g), processed meats, hamburger and bacon (50 g) and pâté (25 g).
After computing total energy intake, a total of 15 matched pairs were excluded due to an unreliable dietary assessment (total energy intake above 4,000 kcal/day), leaving 518 pairs for analysis.
STATISTICAL ANALYSIS
All analyses were performed using Stata (14.0, StataCorp LP, Tx. USA). Qualitative variables were analyzed through frequency distribution, whereas quantitative variables were expressed as means and standard deviation (SD). Pearson2 test and Student's t test were used to assess differences in the characteristics of the participants. All p values are two tailed. Statistical significance was set at p < 0.05. The intake frequencies were derived from the FFQ, thus, each respondent indicated intakes for the different meat groups as: never or more than once serving per week.
Meat and meat products intakes were adjusted for total energy intake using the residuals method as recommended by Willet et al. (39. Food intake was stratified into quintiles, according to intakes observed in the control group (used as criterion of general population intake). This categorization was applied to intakes reported in the SGA group.
Conditional logistic regression models were performed to calculate crude odds ratios (cORs) and adjusted odds ratios (aORs) at 95% confidence interval (CI). The lowest quintile (Q1) was taken as the reference. Intermediate variables were discarded and to control for confounding variables previously analyzed in a priori approach related to maternal diet. The models were adjusted for energy intake, smoking, previous preterm-low birth weight, BMI, newborn's gender and adherence to MedDiet.
RESULTS
One thousand and thirty-six women participated in this study. Table 1 shows characteristics among SGA and control participants. Women in the SGA group were more likely to be smokers and have previous preterm or low-birth weight newborn, preeclampsia and intrauterine growth retardation (p < 0.001). In contrast, more women in the control group were married (p < 0.036) and had a higher mean gestational weight gain and BMI prior to gestation (p < 0.001). No significant differences were observed with regard to education level, Kessner index and alcohol intake.
Table I Description of the study population characteristics in the study (n = 1,036)

SGA: small for gestational age; AGA: adequate for gestational age; SD: standard deviation; BMI: body mass index. Pearson Chi-square test and Student's t test were performed for categorical and continuous variables respectively.
The frequency of intake of different types of meat and meat products and its effect on SGA are shown in Table 2. No significant association was found between the risk of SGA and the intake of most of them. The intake of chicken without skin more than twice a week and a frequent intake of cooked ham (once a day) yielded a lower risk of SGA in crude models (cOR = 0.65; 95% CI, 0.44-0.98; cOR = 0.48; 95% CI, 0.26-0.87, respectively), but both associations disappear in adjusted models.
Table II Frequency of maternal intake of different types of meat and meat products and risk of SGA newborn (n = 1,036)
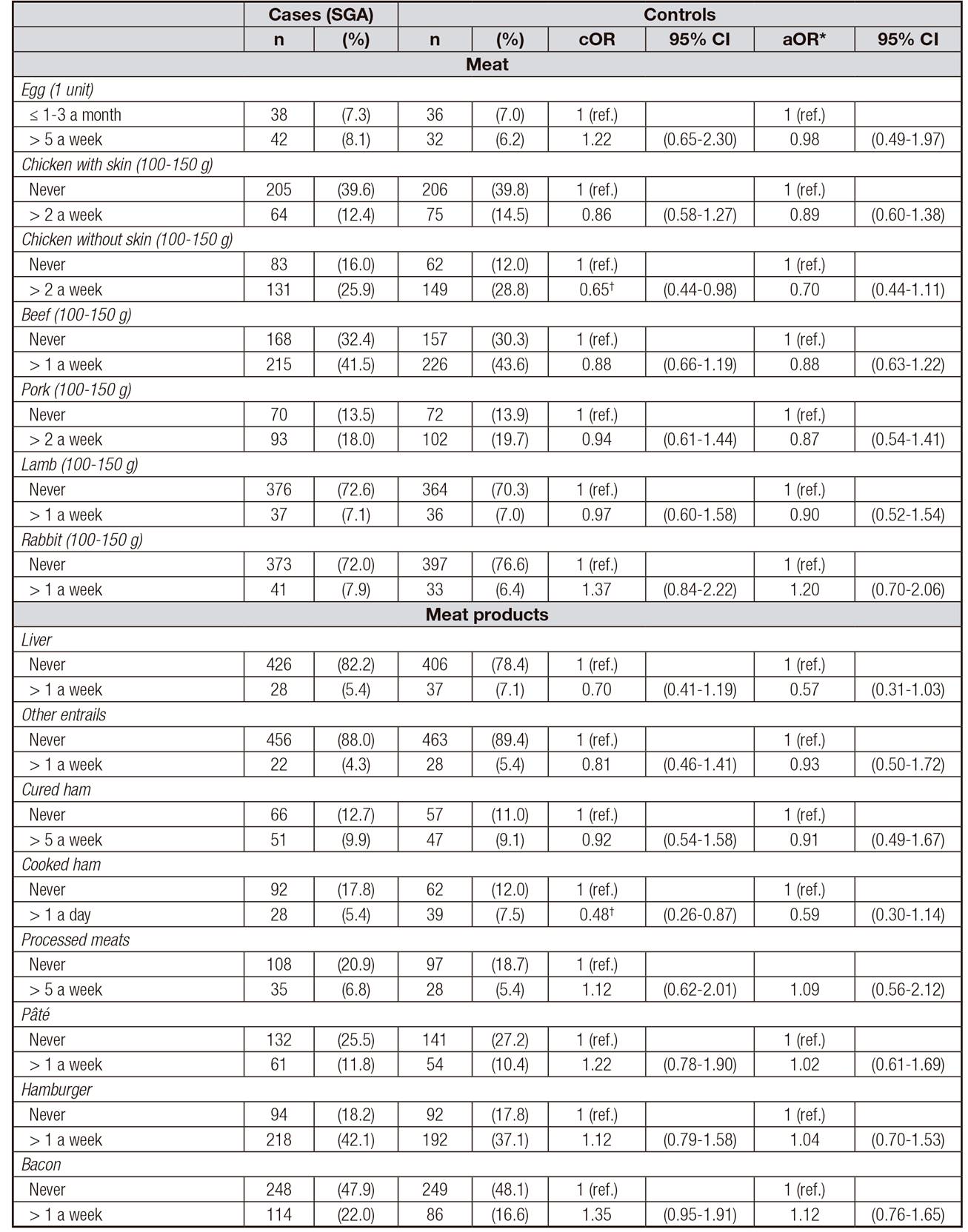
cOR: crude odds ratio; aOR: adjusted odds ratio. *Adjusted for education level, pre-pregnancy body mass index, smoking, previous preterm/low birth weight newborn, newborn's gender, and MedDiet adherence. †Statistically significant (p < 0.05) association.
The relationships between total mean intake of meat and meat products, measured in quintiles (g/day), are presented in Table 3. No significant association was detected with total mean intake and no trend was observed, either in crude results or in adjusted models. Regarding the intake of meat products, no association was observed in raw results. In adjusted results, a significant OR was observed for an isolated quintile (Q4 vs Q1). Given that the OR figures for quintiles Q3, Q4, and Q5 were quite similar, Q3-Q5 vs Q1-Q2 were compared, that is an intake above 6.8 g/d vs a lower one, and the resulting OR achieved statistical significant (aOR = 0.70; 95% CI, 0.53-0.93, p = 0.015).
Table III Daily maternal intake (g/day) grouped by quintiles (Q) of meat and meat products and risk of SGA newborn (n = 1,036)

cOR: crude odds ratio; aOR: adjusted odds ratio. *Adjusted for education level, pre-pregnancy body mass index, smoking, previous preterm/low birth weight newborn, newborn's gender, and MedDiet adherence. †Statistically significant (p < 0.05) association.
DISCUSSION
In the present study, the association between the maternal intake of meat and meat products during pregnancy and the risk of having a SGA baby in a Spanish population was investigated. No significant association for total meat intake was found, although an intake above 6.8 g/day of meat products reduced the risk of having a SGA newborn.
Current pregnancy dietary guidelines are based on dietary reference intakes (DRI) recommendations; protein intake across pregnancy should be upper than in non-pregnant women (around 71.0 g/day) 40. Protein intake in late pregnancy has been traditionally associated with a reduced risk of SGA birth and other maternal and fetal complications 18. For this reason, the general advice is that pregnant women eat more protein, specifically from high biologic value protein sources 41. In our study, the reference group, first quintile, has a mean intake of meat recorded below 99.6 g/day, nearly 30 g above the recommendation. This finding is consistent with a previous research which reported that Spanish pregnant women have a high intake of protein from meat sources 42. The association between meat intake and SGA has been investigated, showing controversial results. Some recent works carried out in European countries (France and Italy) have revealed that a diet in pregnancy based on meat was significantly associated with increased risk for SGA 26,27, whereas other authors have pointed out that mothers of AGA infants ate more servings of meat compared to SGA mothers during pregnancy 28. No detectable effect on meat intake over SGA risk has been seen in this study. Our result may be explained because the lowest quintile intake was above the recommendations, so the reference group is linked to low risk. If this low risk group is located under a saturation line, further increases in meat intake could not show additional risk reductions.
After adjusting by potential confounding factors, no significant association was found when each item was analyzed. However, when stratifying meat products by quintiles a protective effect comparing quintiles 3 to 5 versus 1-2 quintiles was found. This kind of food is typically associated to a western dietary pattern, and has been related to a higher risk of SGA 43; however, this is not supported by our data, possibly because in our population the reference group shows very low intakes, under 7 g/d, and the intakes for upper quintiles are also low. The most frequent advice in pregnancy diet is to avoid raw or undercooked meat products in order to prevent infectious foodborne diseases. Our results do not show any pernicious effect of meat products on the risk of SGA.
The current study has some limitations. Firstly, it is difficult to separate out the specific effects of single food (meat and meat products) on SGA risk because of the highly interrelated nature of dietary exposures; however, our results are adjusted by total energy and diet quality (Mediterranean diet adherence). Secondly, we assessed food intake after birth, so the information registered might not be representative of dietary habits throughout pregnancy time. However, the last gestational dietary patterns could be stable during pregnancy stage 44. Thirdly, although food intake was adjusted by energy consumption and other confounding factors, some residual confounding cannot be excluded. And fourth, a misclassification bias may have occurred. In this sense the information was gathered by midwives, and given that there is no definite knowledge on dietary risk factors and SGA, this bias would be similar in both groups (i.e., non-differential bias), shifting the strength of association toward the null value.
Our study includes some strengths. It includes a vast representative sample of a reference population (around 12,000 healthy pregnant women attending Andalusian public hospitals). In addition, established Spanish fetal growth curves to define SGA have been used 30 and diet was collected throughout a FFQ validated in the Spanish population 35,45. Finally, the control group was sampled by density in the same hospitals (to avoid influence of season in diet reporting).
The present case-control study in Spanish women showed no effect of meat consumption over SGA risk, and a moderate protective effect of meat products intake. These findings support the advice of a varied diet for pregnant women, providing the intake of protein and other micronutrients from different food sources.














