INTRODUCTION
Caseins are the main milk proteins; they constitute a heterogeneous group of phosphoproteins present as stable calcium phosphate-protein complexes named micelles. Their biological functions include supplying phosphate (PO4), calcium (Ca2+) and proteins to neonate, and the release of peptides with biological activity 1. Casein-derived peptides can be liberated both in vivo via normal digestion of casein, as well as in vitro via enzymatic hydrolysis. These peptides have been shown to enhance intestinal maturation in newborns with symptoms of infantile diarrhea or necrotizing colitis 2. Additionally, they can stimulate insulin secretion and lower postprandial blood glucose levels, which demonstrates their relevance for diabetes 2 prevention and management 3. Moreover, some casein-derived peptides are high-energy phenylalanine-free food sources that can play a unique role on nourishing and preventing major neurocognitive deficits on patients with phenylketonuria (PKU) 4.
Phenylketonuria (PKU) is a hereditary metabolic disorder caused by a deficiency of hepatic phenylalanine hydroxylase, which converts the amino acid phenylalanine into tyrosine. Classical phenylketonuria has an international average incidence of 1:11,000 living newborns. If left untreated, the patient will present high phenylalanine concentrations in blood and tissues. This results in severe clinical manifestations such as mental retardation, epilepsy, and behavioral problems. Early detection and strict dietary control is thus essential for those patients 5,6,7,8.
The goal of a treatment with dietary restriction is to maintain blood phenylalanine concentrations within defined target limits, which may vary from country to country 7. The diet should be supplemented with formulas composed of mixtures of amino acids and/or derivatives of proteins in which phenylalanine has been reduced or excluded. Several phenylalanine-free formulas are available in the market. They are a mixture of amino acids plus carbohydrates, vitamins, minerals, and other ingredients, depending on the formula. Usually, they come in powder form and must be reconstituted in water according to the amount recommended by the health professional who monitors the patient. The treatments available may affect absorption of trace elements due to the fact that those diets are poor in animal protein and rich in plant fibers and phytates 9.
Therefore, the nutritional management of PKU would greatly benefit from new dietary options, in addition to new synthetic amino acids formulations that facilitate ingestion of a low-phenylalanine protein source throughout the day 10. Our objective is to explore the potential of casein-derived peptides as an option for low-phenylalanine diets and to describe the production and characterization of these peptides.
MATERIALS AND METHODS
MATERIALS
Casein-derived peptides were obtained from sodium caseinate (Lactonat HV, Lactoprot Deutschland GmbH), with the following composition: protein: 87.5%; moisture: 5.8%; ash: 5.3%; fat: 1.7%; lactose: 0.3%; and pH: 6.6. The Pancreatic Trypsin Novo (PTN 6.0S) enzyme was kindly provided by Novozymes (Novozymes A/S, Denmark).
CASEIN DIGESTION
Peptides were produced by three different methods, although under the same hydrolysis conditions. In the first process, sodium caseinate was hydrolyzed by trypsin with an enzyme to substrate ratio of 1:100, for 240 minutes, at pH 8 and at a temperature of 50 °C. Subsequently, the pH was adjusted to 4.64 with 2M HCl. The insoluble precipitate form was removed via centrifugation at 3,000 xg for ten minutes. This procedure aimed to remove the hydrophobic peptides from protein hydrolysate. CaCl2 (1%) and ethanol (50%) were added to the supernatant; then the precipitate was collected by centrifugation at 4,000 xg for 15 minutes. These peptides were lyophilized and named peptide A (PA).
For the second process, the same procedures were performed as described for PA, but after the addition of CaCl2, the solution was ultrafiltered through a 10 kDa membrane. The pH of the retentate was reduced to 3.55. The product was submitted to diafiltration (1 kDa) with water and concentrated. Thereafter, the pH was raised to 7. The product was lyophilized and named peptide B (PB).
For the third process, the initial steps, which correspond to an acid precipitation, were similar to the previous ones, however 1.1% ZnSO4 was added in order to promote the aggregation of peptides to zinc. The solution was ultrafiltered through a 10 kDa membrane, diafiltered (1 kDa) and concentrated. The product was then lyophilized and named peptide C (PC).
CHARACTERIZATION OF CASEIN PEPTIDES
Composition
Samples were analyzed for nitrogen content by micro-Kjeldahl. Protein content was calculated by multiplying the nitrogen content by 6.25. Ashes were measured via incineration method at 450 °C. Phosphorus content was determined by the molybdovanadate colorimetric method, procedures 22.042-2045 11.
Amino acids
For the determination of amino acids, samples were hydrolyzed at 110 °C in the presence of 6M HCl for 22 hours. By the end of this period, the acid had evaporated; the pH was raised to 8 and the volume to 20 ml. Before the analysis, samples were filtered through 0.22 μm membranes. The analysis was performed by a PNA 402 (Protein and Nucleic Acid Analyzer, Applied Biosystems), with borate buffer at pH 9 at 15 °C. The detection was made on 400 nm/500 nm with a 10 mW laser and 500 KHz.
Predicted protein efficiency ratio
Predicted protein efficiency ratio (PER) was calculated according to Alsmeyer, Cunningham and Happich 12.
RESULTS AND DISCUSSION
The chemical analysis of the products (Table 1) revealed that the protein content of PC is about 84% similar to the content of sodium-caseinate, indicating that only a few peptides were removed from the original protein. PA and PB showed higher content of ashes (19-29%) and lower content of proteins (64-67%).
The association of ions to molecules of casein was reviewed by Gaucheron et al. 13. The authors evaluated the binding of different cations (calcium, manganese, zinc, copper and iron) to caseinate and the stability of these associations under different physicochemical conditions.
Table I. Composition of sodium caseinate and casein peptides

Each value is the average ± standard deviation of triplicate analysis.
Some patents suggested the possibility of purification of casein-derived peptides with the use of any divalent or trivalent minerals. Calcium chloride was the preferential base used, but using ferric chloride or zinc sulfate is also possible 14,15,16.
Sample PB, obtained with the use of calcium chloride, presented high level of ashes, indicating high concentration of the mineral calcium due to the fact that it was not dissociated from the product by the end of the process (in the diafiltration).
The production of peptides from the digestion of casein increases the bioavailability of calcium 17. Ingestion of casein and β-casein produces casein phosphopeptides in vivo in the intestinal tract of rats and is associated with a higher concentration of soluble calcium in the distal ileum lumen 18.
Studies of bone mineral status of children and adults with phenylketonuria showed delayed maturation of the skeleton and osteoporosis 19,20. Thus, casein-derived peptides could be used to improve the profile of bone mineralization of those patients by increasing calcium intake 21.
AMINO ACIDS
Table 2 shows the values of amino acid composition of peptides obtained. The amino acid composition of sodium caseinate showed high levels of phenylalanine. The comparison of the amino acid profile of peptides obtained and sodium caseinate demonstrated a significant reduction in the levels of phenylalanine.
Tryptophan was not determined because this amino acid is destroyed during the acid hydrolysis used in the preparation of the samples.
The advantage of using casein-derived peptides, instead hydrolysates treated for the elimination of phenylalanine, is that casein-derived peptides do not suffer high losses of amino acids that could compromise the nutritional value of these compounds.
Bernardi et al. 22 found that the use of a vacuum evaporator to remove HCl promotes loss of threonine and serine, primarily related to destruction by heat. The neutralization with citrate/NaOH reduces these losses. However, the inclusion of salts interferes with the determination by capillary electrophoresis, and for this reason, neutralization was not adopted.
Table II. Amino acid composition (g amino acids/100 g protein)* of casein peptides obtained and commercial sodium caseinate
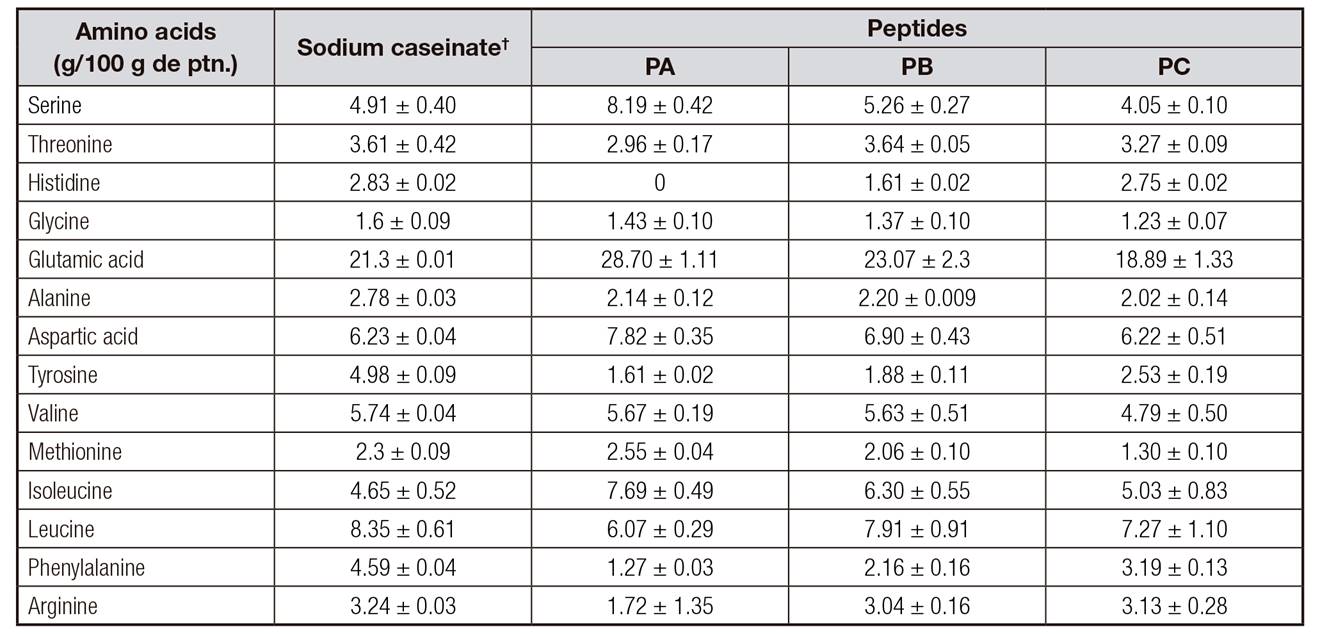
*Values are means ± DP
†Sodium caseinate; Lactoprot, Germany (http://www.lactoprot.de/)
Comparing the amino acids profile of the obtained peptides (Table 2) with the Food and Agriculture Organization/World Health Organization (FAO/WHO) 23 recommendations, an average value of 10 g of protein per day is the recommended protein intake for a three-year-old child weighing approximately 15 kg (0.66 g/kg/day). Essential amino acids analyzed in samples PB and PC were sufficient to meet the amino acid requirements for preschool children and adults 23. PA, PB and PC could contribute significantly to the proper daily intake of non-essential amino acids such as serine, glycine, glutamic acid, alanine, aspartic acid, and arginine.
Amino acid analysis revealed a significant reduction in the amount of phenylalanine (Phe) in the PA. The value for this product represented a reduction of 72.33% compared to sodium caseinate, and of 51.89% and 26.61% for other studies which developed hydrolysates with low phenylalanine 5,24. These studies used charcoal in the adsorption of amino acid, which may cause loss of essential amino acids due to the low specificity of coal.
Peptides PA, PB and PC resulted in products with 12.70 mg, 21.60 mg and 31.90 mg of Phe per gram of protein, respectively. Phenylalanine tolerance is variable among phenylketonurics, depending on their residual enzyme activity. This tolerance ranges between 200 mg/day and 2,000 mg/day. The majority of patients with severe PKU tolerate less than 500 mg/day of phenylalanine. Vegetables and cereals are the main sources of protein in their diets 25. Supposing that the recommendation of daily protein intake for a three-year-old child is 0.66 g/kg/day 23, a formulation that uses the PA peptide will respect the acceptable daily intake of phenylalanine to children with phenylketonuria.
Protein efficiency ratio (PER) is an indicator of protein quality in food. The predicted PER value obtained for sodium-caseinate and the different casein derived peptides accessed based on amino acid analysis were of 2.8 for sodium-caseinate, 2.1 for PA, 2.9 for PB and 2.6 for PC. PER value greater than 2 are well correlated with a protein's adequate capacity to promote physical growth and development. Therefore, all casein-derived peptides seem to be well recommended to patients within various life stages, including those with greater nutritional demand 12,26.
The use of protein hydrolysates containing short-chain peptides is highly desired in dietary formulas. The absorption of short-chain peptides is considered to be more efficient compared to an equivalent amount of free amino acids. This is due to the availability and speed of peptides' specific transport system in the enterocyte, and to their subsequent break into amino acids, caused by the action of cytoplasmic peptidases before transportation to the blood circulation. Moreover, the transport of free amino acids is easily saturable and competitive, decreasing the speed and the rate of absorption 27.
Furthermore, peptides are less hypertonic mixtures of amino acids, facilitating the absorption of other dietary components, thus reducing osmotic problems. Due to chemical instability and insolubility in water, some amino acids, such as glutamine, tyrosine, and cysteine cannot be easily administered in free form 27.
Additionally, peptides derived from proteins of bovine milk have the potential of exerting beneficial physiological effects, hence why they are called bioactive peptides. Anti-hypertensive, anti-inflammatory, antioxidant, immunomodulatory, antimicrobial, opioid agonist, opioid antagonist, and antiproliferative activities are the main functions that can be performed by bioactive casein-derived peptides in different organism systems 28.
A dietary treatment option for PKU patients is the glycomacropeptide (GMP), a protein derived from k-casein via the action of chymosin. This protein is palatable, rich in branched chain amino acids, and it does not present aromatic amino acids (phenylalanine, tryptophan and tyrosine) in its natural composition 29. GMP contains limited amounts of histidine, indispensable for individuals with PKU 30. Commercial highly-purified GMP contains less than 2.0 mg phenylalanine per gram of protein. However, to provide a complete source of protein for individuals with PKU, GMP must be supplemented with arginine, histidine, leucine, tyrosine, and tryptophan 31.
CONCLUSION
The protein efficiency ratio of the obtained peptides demonstrated that they can be used as a main protein source. Therefore, the most important finding of this study was that casein-derived peptides could be used in the future as an important alternative in the preparation of low-phenylalanine formulations.















