INTRODUCTION
Type-2 diabetes mellitus (T2DM) is a major public health problem worldwide. Current pharmacological treatments aim to control serum glucose levels in order to prevent systemic-vascular and neural damage 1. Control of serum glucose levels in T2DM has been associated with improved quality of life and decreased metabolic complications 2. Intensive control of T2DM may require the addition or the increase of doses of oral hypoglycemic drugs or the use of insulin 3. However, these pharmacological strategies pose potential negative consequences for patients, including drug-side effects, increased costs, reduced compliance or even increased risk of mortality due to hypoglycemic events 4,5; these consequences could be even more severe in elderly patients that usually have other comorbidities. In addition, T2DM treatments can be difficult to obtain and are costly. The estimated cost for the treatment of diabetes considering direct and indirect expenses in the United States is 1.31 trillion dollars per year 6. The increasing frequency of chronic diseases in low- and middle-income countries anticipates a very high economic burden for those nations in the coming years 7. Although pharmacologic treatment for T2DM has an important role to control the disease, additional non-pharmacologic complementary treatments are needed to prevent and control this public health problem.
A healthy life style including physical activity, smoking cessation, and therapeutic lifestyle change (TLC) dietary options including consumption of complex carbohydrates, mono- and polyunsaturated fat, and animal or plant derived proteins are associated with better T2DM prevention and control 8. Regarding animal protein consumption, greater dairy products intake results in better glucose regulation and T2DM prevention 9. In addition to protein content, beneficial effects of dairy products can be associated with their micronutrient, fatty acids, low-glycemic carbohydrates, free amino acids, and active peptides content 10. Also, higher plant protein intake has been associated with decreased risk of T2DM 11. Consumption of legumes, grains characteristically rich in proteins, is associated with lower risk of non-cardiovascular and total mortality 12.
Lupin is a legume rich in proteins and widely consumed around the world whose consumption has been associated with decreased blood pressure, improvement of serum lipid profile, positive alterations of gut microbiota and glucose homeostasis 13. Together, these studies indicate that consumption of high-quality proteins as part of TLC could optimize current treatments of non-communicable chronic diseases, including T2DM.
We have demonstrated that Andean Lupinus mutabilis intake decreases blood glucose and improves insulin sensitivity in individuals with dysglycemia or T2DM. Those studies indicate that consumption of either raw as well as cooked L. mutabilis significantly lowered serum glucose and insulin levels in the short term 14,15. In addition, Bertoglio JC et al. have documented the hypoglycemic effect of lupin derived γ-conglutin protein in experimental animals and healthy human subjects 16. We recently demonstrated the potential molecular and cellular mechanisms for the hypoglycemic effects of Andean L. mutabilis Sweet. Hydrolyzed γ-conglutin fraction from Andean L. mutabilis at 5 mg/ml inhibited dipeptidyl peptidase IV activity (100% inhibition), stimulated glucose uptake (6.5 fold) due to GLUT-4 cell surface translocation, and inhibited hepatic gluconeogenesis (50%) in a dual-layered enterocyte/adipocyte/hepatocyte culture system 17. These studies show that lupin proteins and peptides have several properties and their intake has positive effects in glucose metabolism.
There are limited human clinical studies on lupin consumption as complement for the pharmacologic treatment of T2DM. This quasi-experimental 28-week crossover-study aimed to compare the efficacy of the daily consumption of a snack of L. mutabilis on metabolic control of T2DM patients under conventional oral pharmacologic treatment.
METHODS
SUBJECTS AND STUDY DESIGN
This was a quasi-experimental clinical trial with a crossover design in which patients received their usual prescribed oral anti-diabetes treatment for 14-weeks and subsequently, in addition to their oral anti-diabetes treatment they consumed a snack based on L. mutabilis for other 14-weeks.
We recruited patients with T2DM who regularly attended the Paute Hospital near the city of Cuenca or the General Teaching Hospital in the city of Riobamba, in Ecuador.
Ninety-two T2DM patients under conventional oral-hypoglycemic-treatment of both sexes, older than 18 years of age were originally screened for the study. Patients were excluded if they were under insulin treatment, had a value of A1C > 10% either at the baseline-test or during the study, presented severe acute febrile diseases or chronic diseases (such as cancer, human immunodeficiency viral infection, autoimmune diseases or psychiatric diseases), or were not willing to sign an informed consent (Fig. 1).

Figure 1. Flowchart of data analyzed to evaluate the effect of consumption of a Lupinus mutabilis-based snack on T2DM metabolic control.
Initially, patients received a complete nutritional assessment that consisted in a medical evaluation, anthropometric measurements, blood biochemistry analysis, and dietary intake. Patients also provided basal sociodemographic data and they answered the physical activity IPAC questionnaire 18. Patients under their usual treatment were followed for 14 weeks at which point the same measurements done at baseline were performed again. After this second visit, the nutritional intervention with lupin was initiated. During the initial seven-weeks of the intervention-phase, patients continued with their usual drug treatment plus one-daily-dose of 10 g of a L. mutabilis-based snack that was consumed 30 minutes before lunch. During the following seven weeks patients continued with their usual drug treatment plus two daily doses of 10 g of a L. mutabilis-based snack 30 minutes before lunch and 30 minutes before dinner. At the end of the intervention period a final measurement of the study variables was done.
Evaluators of outcomes and data analyst were blinded to the treatments that patients received during the study.
CHARACTERISTICS OF THE SNACK
Lupinus mutabilis grains cultivar 450 were used to prepare a dry crunchy snack by the Instituto Nacional de Investigaciones Agropecuarias (INIAP), Quito-Ecuador. The snack consisted in 10 g of dehydrated L. mutabilis lightly salted and packed in a small bag of aluminum paper which was completely sealed. The macro and micronutrient composition of the snack is presented in Table I 19.
ANTHROPOMETRIC AND BLOOD PRESSURE MEASUREMENTS
Standardized clinic weight and height scales were used to determine both height and weight of each participant. A digital weight scale (Seca®, Quito-Ecuador) calibrated to the nearest 0.1 kg was used to weight patients. For height measurement, a portable stadiometer (Seca®) with a precision of 1 mm was used. Weight and height were taken with light clothing and no shoes 20. Blood pressure measurements were taken by a trained member of the research team using an automatic digital monitor (Omron HEM-757; Omron Corp., Tokyo, Japan); two consecutive recordings of blood pressure were taken after five minutes of rest in a sitting position. Two readings were obtained and the mean value was used for analysis.
BLOOD SAMPLES
Qualified personnel took blood samples in the morning between 8 a.m. and 10 a.m. after approximately 12 hours of fasting. Samples were centrifuged within two hours and processed immediately. Samples were collected at baseline, 14 weeks and 28 weeks.
Serum glucose was measured using a glucose oxidase method (Roche-Diagnostics, Quito, Ecuador) using a Hitachi Roche 917 full-automated analyzer system. Serum insulin was determined using an electro-chemiluminescence immunoassay (ECLIA) following the manufacturer's instructions (Roche Diagnostics/Hitachi, Quito, Ecuador) and chemiluminescent emission was measured using a fully automated analyzer system ELECSYS 2010 (Roche Diagnostics, Quito, Ecuador). Both glucose and insulin determinations were carried out in NetLab Laboratory. NetLab maintains an internal and external quality control system (College of American Pathologists, Brazilian Society of Clinical Pathologists, SBOC).
The primary end points of the study were the changes in A1C during the study periods (14 weeks vs 28 weeks and baseline vs 28 weeks). Secondary end-points included: changes in body mass index (BMI), blood pressure, insulin and lipid profile.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Human Subjects Protection Committee at the Universidad San Francisco de Quito approved this study. Each participant signed an informed consent form after receiving an explanation of the study and its possible consequences.
SAMPLE SIZE AND STATISTICAL ANALYSIS
A sample size of 63 patients was determined to have 80% power to detect a statistically significant decrease in the final A1C value from 8.1% at baseline to 7.7% at 28 weeks, assuming a standard deviation of 0.8. Since this was a quasi-experimental study, patients served as their own control during the two-phases of the study.
Descriptive statistics such as percentages and means with their standard deviation were calculated. To assess changes from baseline a paired t-test analysis was conducted. A p-value ≤ 0.05 was considered as statistically significant after the Benjamini-Hochberg correction 21.
RESULTS
A total of 79/92 (85.9%) patients who met the eligibility criteria were enrolled in the study. Twenty-eight patients were excluded during the development of the study because they either dropped the study (n = 15) or presented A1C levels > 10% (n = 13). Out of these 13 patients, eight had serum A1C levels greater than 10%, after the first phase of the study (without lupin consumption) and five patients at the end of the study period (after lupin consumption); these five last patients maintained serum A1C levels greater than 8% throughout the study period.
Lupinus mutabilis snack was well accepted and tolerated by all participants. Approximately 83% of patients reported a perfect adherence to snack consumption during the study. The remaining 17% also had a good reported adherence (more than 75% of snack was consumed), however, they did not take all the scheduled snacks due to forgiveness or denture poor conditions that made it difficult to chew the snack.
SOCIODEMOGRAPHIC CHARACTERISTICS
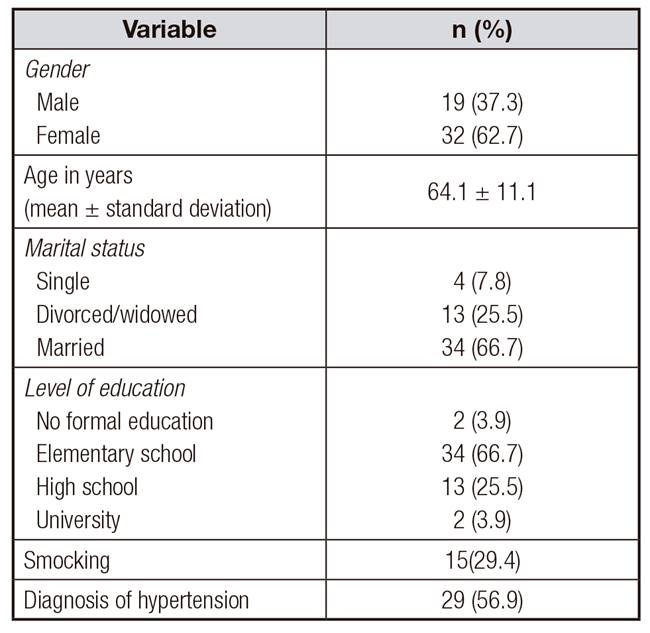
Table II shows sociodemographic characteristics of participating patients. There were more women than men and participants age ranged between 35 and 88 years. Most participating patients were married, with elementary school level of education. An important percentage of patients smoked and presented hypertension.
ANTHROPOMETRIC AND BLOOD PRESSURE CHANGES
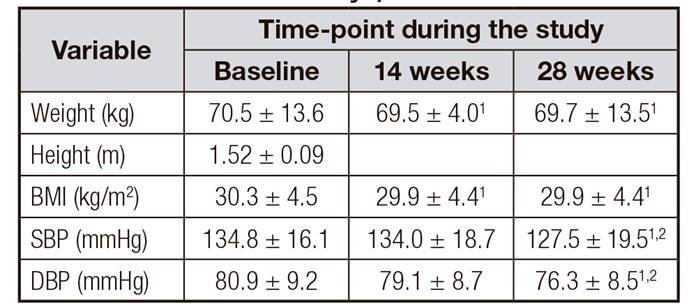
There was a statistically significant decrease in weight and in BMI from baseline to the end of the study at 28 weeks, p = 0.015 and 0.009, respectively (Table III). However, these two parameters did not changed significantly from the 14 to the 28 weeks. In addition, both systolic and diastolic blood pressure were significantly lower at 28 weeks after the supplementation with L. mutabilis compared with 14-weeks and baseline measurements.
CHANGES IN BLOOD BIOCHEMICAL PARAMETERS
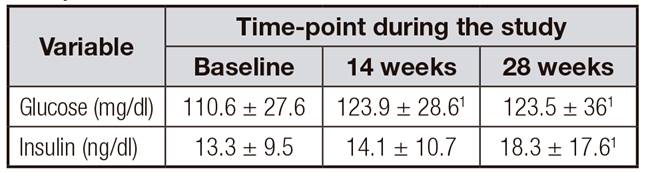
As shown in Table IV, there was a significant increase of glucose and insulin from baseline until the end of the study but there were not important changes between 14 and 28 weeks. Serum A1C levels did not change significantly during the study. In addition, serum HDL concentrations increased significantly at 28 weeks compared with baseline. No other serum markers of lipid metabolism, uric acid or CRP showed statistically significant modifications during the study.
Table IV. Changes in blood biochemical parameters during the study

1Different from baseline.
2Different from 14 weeks.
A closer analysis in patients with levels of A1C ≥ 8.0 and ≤ 10 showed that there were no statistically significant changes in A1C levels throughout the study period (Table V).
Table V. Changes in A1C and percentage of patients that achieved treatment target value according to the severity of metabolic alterations

1Different from baseline.
2Different from 14 weeks.
However, it is noticeable that patients who maintained their A1C levels ≤ 8.0% reduced significantly their levels of A1C after the consumption of the snack achieving a reduction of 0.2-0.4% by the end of the study. Although not statistically significant, only those patients who maintained their A1C serum levels ≤ 8.0% more frequently reached the treatment target value of A1C of 6.5% (Table 5). In addition, in this subgroup of patients, glucose increase by 14 weeks was attenuated after lupin consumption. This increase in glucose was accompanied by an increase in serum insulin that was greater with lupin intake (Table VI).
DISCUSSION
The present study demonstrated that consumption of a snack based on the legume L. mutabilis by T2DM patients under regular hypoglycemic treatment improved glucose metabolic control. Thus, patients with serum A1C concentrations ≤ 8.0% that consumed the snack more frequently achieved the ideal target of 6.5% A1C 1. The lower the level of A1C patients maintained, the grater the benefit of the addition of the snack of L. mutabilis. This study also showed a significant decrease in BMI and blood pressure and a significant increase in HDL cholesterol by the end of the study period.
A limited number of studies with humans have documented the hypoglycemic effect of L. mutabilis consumption in T2DM patients, although those reports are restricted to short-term interventions 14,15,16. The present report expands those results and demonstrated that consumption of L. mutabilis for 14 weeks improved glucose metabolism reflected in lower concentrations of A1C in patients with serum A1C levels ≤ 8.0%. In these patients, the typical decrease in A1C after 14 weeks of L. mutabilis consumption was 0.2-0.4%, which represents a diminution of about 10 mg/dl of serum glucose. Most oral hypoglycemic drugs lower A1C within a range of 0.5 and 1.5% 1. Patients with T2DM that maintained abnormal serum levels of A1C ≤ 8.0%% could benefit with the addition of a snack of L. mutabilis to their conventional oral glucose lowering treatment. It looks like the greatest benefit of L. mutabilis consumption by T2DM patients depends on the severity of their metabolic alteration manifested by A1C serum concentrations. On the other hand, patients with serum A1C levels > 8.0% did not show significant improvements of glucose metabolism. It is important to note that during the first 14 weeks of the study, when patients did not receive L. mutabilis there was an increment in blood glucose that was attenuated after L. mutabilis consumption in patients with A1C serum levels ≤ 8.0%. As expected, the observed increments in glucose were paralleled with greater concentrations of serum insulin; insulin increases were greater after L. mutabilis consumption. In this regard, oral administration of γ-conglutin from lupin (120 mg/kg) during one week increased the expression of Ins-1 gene and pancreatin insulin content in an animal model for diabetes 22. It is possible that hydrolysates from L. mutabilis (including those derived from γ-conglutin) could have increased insulin production directly stimulating pancreatic b-cells or through the increase of GLP-1 produced by inhibition of dipeptidyl peptidase IV (DPP-IV) activity 17. These studies indicate that the consumption of L. mutabilis could increase serum insulin concentrations as observed in the present study.
It is important to consider that L. mutabilis is a high-quality food which provides other important health benefits; it is well accepted and affordable, it is feasible to increase its daily dosage and does not cause side-effects nor has negative interactions with other drugs unlike most hypoglycemic medications. The mechanisms of action related to the consumption of lupin and other legumes are under intensive study 16,17,18,19. We have shown that hydrolysates of L. mutabilis inhibit gluconeogenesis, DPP-IV, and enhance the sensitivity of the insulin receptor mediated by translocation of the transporter of GLUT-4 17. These molecular and cellular mechanisms resemble the biochemical effects of sitagliptin (gliptins) and metformin drugs (biguanides).
Previous studies have reported a decrease of 2.4 to 8.4 mmHg in systolic blood pressure 23,24 after consumption of 15 to 25 g/day of L. angustifolius protein isolate for 12 month and eight weeks, respectively 23,24. In agreement with those observations, here we demonstrated that upon consumption of L. mutabilis there was a decrease of 7 and 3 mmHg in systolic and diastolic blood pressures, respectively. A possible mechanism of action of lupin consumption on blood pressure could be related to its important content of protein and fiber and the dietary substitution of carbohydrate with them 25,26,27. Also, amino acid sequences of hydrolysates generated after L. mutabilis protein digestion could have potential inhibition of the angiotensin converting enzyme 17. A reduction of 7 mmHg would reduce cardiovascular risk by about 14% 25. In addition, the observed increase in HDL-cholesterol in this study could further contribute to decrease the cardiovascular risk with L. mutabilis consumption. The present results agree with other observations that demonstrate increases in serum HDL of subjects with hypercholesterolemia after consumption of lupin protein isolates from L. angostifolius 23. It is difficult to estimate the magnitude of reduction in cardiovascular risk because there is controversy regarding the benefit of increasing HDL levels 28. Interestingly, recent epidemiological studies demonstrate that consumption of legumes is inversely associated with total mortality and non-cardiovascular death 29. In addition, these studies indicate that lupin species intake can positively affect cardiovascular risk factors in at risk populations.
Limitations of the present study included the low number of eligible patients: 51 instead of the initially calculated sample of sixty-three. This could have precluded to achieve statistical significance of the effect of the consumption of L. mutabilis in subjects that regularly have A1C levels > 8%. Future studies should assess higher doses of L. mutabilis intake, the effects in glucose metabolism in subjects with A1C levels > 8% and, most important, the potential of this legume to prevent T2DM.
CONCLUSIONS
Therapeutic dietary changes including frequent consumption of legumes are promising complementary strategies for the management of patients with T2DM. In this quasi-experimental crossover study, an intervention with 15 g of LM resulted in a significant decrease in blood pressure and a significant increase in HDL-cholesterol. Patients with serum A1C concentrations < 8.0% reduced significantly their A1C after the intervention and 71.1% achieved a target concentration ≤ 6.5%. Patients with T2DM could benefit with the addition of LM-snack to their conventional treatment.