INTRODUCTION
Vitamin D is attributed to calcium and phosphate metabolism 1,2,3. At the same time, it is also involved in cell differentiation, proliferation and immunomodulation 4. Its requirement for the organism is basically supplied by endogenous synthesis (90-95%). However, endogenous synthesis is profoundly affected by several factors, such as the degree of skin pigmentation, the time of the day of exposure to the sun, season, weather conditions, air pollution, clothing and the use of sunscreen 3.
Vitamin D deficiency is characterized by serum levels below 20 ng/mL; insufficiency, between 21 and 29 ng/mL; and adequacy, serum levels greater than 30 ng/mL 5,6. Deficiency and insufficiency are associated with several pathological conditions such as lack of exposure to UVB, inadequate dietary intake, malabsorption, multiple risk factors for CNCD 7.
The relationship between chronic viral infections and hypovitaminosis D is well known. A high prevalence of vitamin D deficiency was specifically described in the study by Villamor 8 in the seropositive population, with high rates of 83%, corroborating with the EuroSIDA study 9. Several studies have focused on the parameters that influence hypovitaminosis D in HIV-positive patients 10.
However, the results of the analyzes of many studies around the world remain discordant, especially regarding the modalities of treatment. In fact, several studies have shown that vitamin D concentrations are related to antiretroviral therapy (ART) and to the use of specific antiretroviral class.
The concentration of vitamin D may be specifically lower in patients treated with non-nucleoside reverse transcriptase inhibitors (NNRTIs) than in those treated with protease inhibitors (PIs), in particular efavirenz, but there are still discrepancies on the deleterious role of NNRTI regarding levels of vitamin D 11. About the severity of the disease, vitamin D deficiency is associated with a low TCD4 cell count and a viral load higher than 50 copies/mL 12. In addition, vitamin D deficiency is more common in HIV-infected patients with advanced pathology progression 13. Thus, there is an increased risk of morbidity and mortality in HIV-infected patients with low levels of vitamin D 14.
Scientific evidence involving vitamin D supplementation in people with human immunodeficiency virus is very few and inconclusive, so the aim of this study was to conduct a systematic review of the available clinical trials on this subject, in order to lead researchers to this promising theme in the field of nutrition.
METHODS
SEARCH STRATEGY
A systematic review (RS) of clinical trials about vitamin D supplementation in HIV patients was performed. In the elaboration of the guiding question of the present study the PICO strategy was used, which represents the acronym for problem or population (P), intervention (I), comparison (C) and outcome (O).
The PICO strategy culminated in the delimitation of the following guiding question: does vitamin D supplementation lead to improvements in the clinical picture of HIV patients? Each PICO dimension was equivalent to the following elements: (P) HIV patients, (I) vitamin D supplementation, (C) placebo, and (O) clinical improvement of HIV patients.
The virtual search was performed in the PubMed, Scopus, Science Diretc, Scielo, LILACS and Cochrane Library databases, from February to April 2018, by two authors. The researched clinical trials had publication limits between 2000 and 2018, without gender, ethnicity restrictions, and published in the English language, involving individuals aged over 18 years. The "and" connective was used in the combination of the Medical Subject Headings Terms (MeSH terms): vitamin D, supplementation and HIV.
SELECTION OF PAPERS
The titles and abstracts of the selected articles were analyzed to evaluate whether they met the inclusion criteria: to have a controlled clinical trial design and to be available in full. The evaluation of the eligibility criteria was done independently by two authors, and in case of divergence a third researcher was consulted.
QUALITY ASSESSMENT OF STUDIES
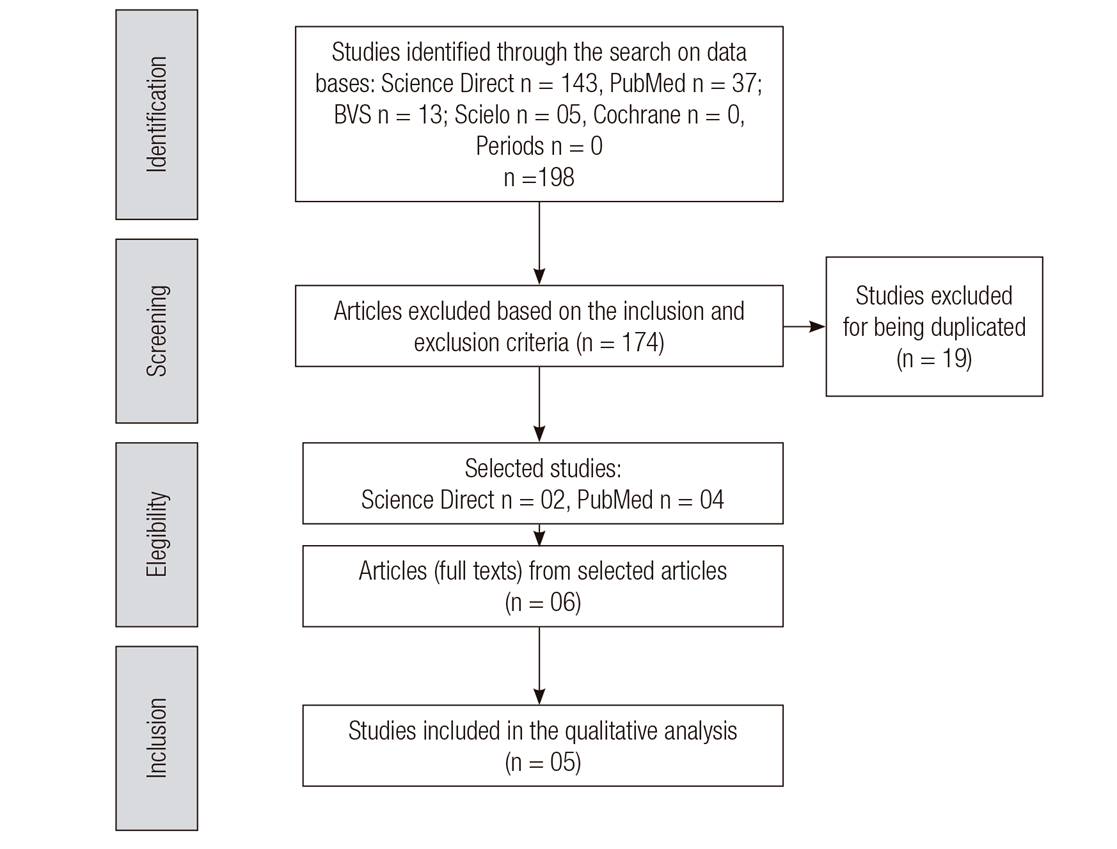
In order to assure the quality of the SR, the protocol Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) was used 15. The Jadad scale 16 was employed independently and blinded by two researchers for qualitative classification. Scores were assigned to the studies (from zero to five), based on the criteria: randomization method (sequences and criteria for randomization of participants), use of masking (for patients and researchers) and description of the proportion of loss to follow-up. The risk of bias in the trials included in this study was identified by the Cochrane Collaboration Tool 17 (Fig. 1).
RESULTS
The bibliographic research, according to the pre-established strategy, initially resulted in 198 articles. Of these, 143 from Science direct, 37 studies from the PubMed database, 13 from the VHL, 05 from Scielo and no articles from Periods and the Cochrane Library. After the process of selection and removal of 19 articles, that were duplicated and that were not clinical trials, 05 original articles from randomized controlled trials (RCTs) dealing with vitamin D supplementation in adults living with HIV were identified as eligible for this systematic review. Figure 1 shows the flowchart of the result of the search in the sources of information, of the selection and inclusion of the original articles in the Systematic Review, according to the protocol PRISMA statement.
Clinical trials showed homogeneous methodological quality based on bias risk assessment by the Cochrane tool (Table I). Random sequence generation was adequately reported in 40% (2/5) low risk and 60% (3/5) uncertainty studies, allocation concealment with 100% (5/5) low risk, blinding of participants and professionals 100% (5/5) low-risk, blinding of outcome assessors with 20% (1/5) of uncertain and 80% (4/5) of low risk for incomplete outcomes were 40 (2/5) of uncertain 60% (3/5) of low risk, in the report of selective outcome we had 100% (5/5) of low risk and other sources of bias presented 100% (5/5) of low risk.
Table I. Analysis of the methodological quality and risk of bias proposed by the Cochrane Collaboration. Teresina-PI. Brazil, 2018
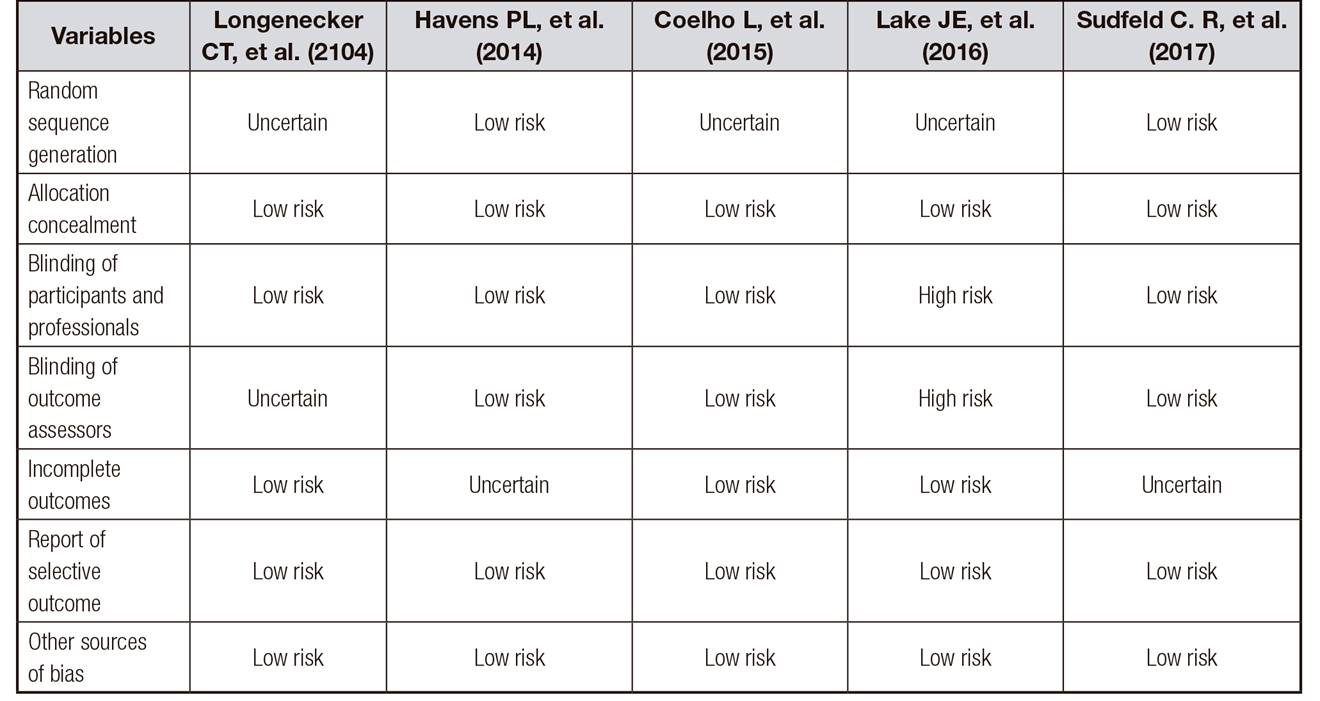
Table II shows the results of the quality evaluation of articles analyzed according to the Jadad scale. In relation to the items evaluated, all articles adequately described the aspects evaluated in that scale.
Table II. Characteristics of studies according to authors, country and year of publication, sample, analyzed variables, intervention and outcome in people living with HIV supplemented with vitamin D. Teresina-PI. Brazil, 2018


The data presented in Table II combine the results of the reviewed articles including authors, year of publication, place of study, study sample size, dose, duration of supplementation and main outcomes. It was observed that the five trials included in this review were conducted with persons over eighteen years of age of both sexes, in different countries. Doses of vitamin D supplementation ranged from 2,000 IU to 50,000 IU per week, and the duration of interventions ranged from 12 weeks to 3 years.
The main variables investigated were: 25(OH)D, socio-demographic data, age, BMI, CD4 lymphocytes, but some studies went further and included biochemical tests and immunological data.
The results of the five clinical trials demonstrated that vitamin D supplementation increased serum levels of the vitamin. In addition, they demonstrated a positive effect of supplementation on CD4 lymphocytes count supporting the vitamin D benefit in immunological recovery, which is particularly relevant for the HIV-infected population and effective for a variety of antiretroviral suppressive regimens (ART).
DISCUSSION
Antiretroviral therapy coverage is rapidly expanding worldwide and treatment programs need interventions in order to prolong and improve the quality of life of HIV-infected individuals in resource-limited contexts 18. Supplementation of vitamin D3 may be an associated and effective intervention since vitamin D3 deficiency is common among HIV-infected individuals, ART may reduce even more vitamin D levels by altering their metabolism. Multiple cohort studies have determined that low levels of vitamin D are associated with increased risk of mortality, tuberculosis and disease progression among individuals and supplementation is known to be effective and safe in improving their condition 8,19,20.
About the extra skeletal functions of vitamin D, the presence of a nuclear vitamin D receptor in many human tissues has been described, corroborating with its current definition of hormone 21. The deficiency of this substance is being increasingly described in the world population and attributed to this, its association with the development of immunological, neoplastic, endocrine-metabolic diseases, among others. However, there is a great discussion as to the serum levels of vitamin D considered insufficient or deficient, hindering the conduct of supplemental therapy.
Despite the high rates of vitamin D insufficiency in patients on antiretroviral therapy, few studies have been carried out regarding the supplementation of this vitamin in this population, here in Brazil. In other countries this topic is growing among the researches, with important associations with the anthropometric, biochemical and immunological parameters in this population. The included articles resulted in a population of 4,470 people living with HIV. Regarding the gender, two articles had a majority of males, and in regard to ethnicity the majority of white people. As for the age group, the selected studies had similar average ages of 45 22 and 49 years 23.
Therefore, the studied population with an age group of young adults falls within the growing risk group among HIV-positive individuals, being a group at risk for having or acquiring vitamin D deficiency and cardiovascular diseases 24, which makes the analysis of these variables with vitamin D deficiency an important study to understand metabolic changes that may occur in this public. This study has a limitation regarding the sample that would be small to fully evaluate the relations between the level changes of 25(OH)D and specific clinical and demographic factors. But Sudfeld et al. 25 brings a very high number of participants, confirming that vitamin D deficiency among HIV-infected individuals has listed mortality or morbidity outcomes.
The research by Coelho et al. 23 was carried out in Rio de Janeiro, during 2 years and throughout all seasons, similar to the one by Lake et al. 22 that carried out in Los Angeles, United States, for 11 months, as well as the other studies that took seasons into consideration.
In North America the seasons are well defined, differently of what happens in South America and Africa, which can influence on the results. It is important to consider the seasons in the studies with vitamin D, considering that the concentrations of this vitamin can be altered according to the solar exposure and radiation to which each person will be exposed, possibly being a variable of confusion 24.
Body composition is of extreme importance for assessing the prognosis of HIV/AIDS, as well as the nutritional status, which interferes in a directly proportional way in vitamin D concentrations 26. Among the articles analyzed, all of them presented a population that was mostly eutrophic according to the body mass index (BMI), however this index alone is not a very effective method for analyzing body composition. It is important that their values correlate with other independent measures that determine the composition in terms of body fat and lean mass 27. Therefore, the assessment of body composition is recommended to have BMI, waist circumference, skin folds, being quite effective.
CD4 + or T-helper cells are leukocytes that organize the immune system response to some microorganisms, including viruses. The CD4 + cell count in a healthy, non-HIV-infected individual may range from 500 to 1,200 cells/mm3. This quantification assesses the state of the immunological system and the risk of debilitating complications and infections. It is known that the human immunodeficiency virus infects CD4+ cells and decreases their available amount in the human body. A low concentration of these cells, from 200 to 250 cells/mm3, increases the risk of opportunistic diseases 28.
In the study by Lake et al. 2267% of the participants were in the range of 200 to 499 cells/mm3, while in the one by Coelho et al. 23 72% were in the range > 500 cells/mm3, such as on the studies by Legeai et al. 29 and by Sheperd et al. 30 who demonstrated that there is an association between vitamin D levels and the number of CD4 + cells, and that these decreased levels would be related to inflammation in untreated HIV patients, which has led to researches with vitamin D supplementation, aiming to reduce the level of infections of these individuals.
In HIV treatment programs in Tanzania and sub-Saharan Africa they are moving towards the smaller use of TCD4 cells from 350 to 500 with μL limit for initiation of antiretroviral (ART) based on WHO guidelines 31.
As Wagner et al. 32 reported, there is no consensus on the exact values of normal concentrations of vitamin D, and there is no standardization of methodologies to assess its deficiency, insufficiency and sufficiency, so the comparison between studies that used different cut-off points for this vitamin should take into account the different measurement parameters 33.
Although calcidiol is recognized as an ideal parameter for evaluating body vitamin D, there is no agreement between the organizations or authors regarding the ideal cut-off point for characterizing vitamin D deficiency, as it was observed in the results of this article, in which the authors used different cut-off points to classify sufficiency and insufficiency. The authors Lake et al. 22 and Coelho et al. 23 classified vitamin D sufficiency and deficiency in which the 25(OH)D levels were ≥ 30 ng/mL and < 30 ng/mL. In the study by Sudfeld et al. 25 the restriction of the studied population for subjects was less than 30 ng/mL of 25(OH)D that also assists in translating the findings as HIV Programs in similar environments that may estimate the potential impact of implementation of vitamin D3 supplementation based on the local prevalence of 25(OH)D < 30 ng/mL.
Several HIV related risk factors, as well as non-HIV related factors, have been associated with vitamin D deficiency 34. Some risk factors such as winter, increased age, low daily vitamin D intake, and elevated skin pigmentation are common to the general population. In addition to the risk factors observed in the general population, several studies have demonstrated an association with highly active antiretroviral therapy (HAART), especially with the use of efavirenz, a NNRTI, with increased risk of vitamin D deficiency 35,36.
Participants in the selected studies in this review were using (HAART). In the study by Coelho et al. 23, the levels of 25(OH)D were decreased within 24 weeks after initiation of efavirenz with lamivudine/zidovudine or emtricitabine/tenofovir, but they stabilized thereafter 36. With an average time of five-year therapy, the authors were not able to assess changes in the 25(OH)D levels related to the initiation of HAART, however, vitamin D insufficiency was associated with current use of efavirenz and nevirapine regardless of the duration of the treatment.
At the study by Lake et al. 22 no association was observed between specific antiretroviral agents and basal levels of 25(OH)D, as there was no association between antiretroviral agents and the success or failure to reach levels higher than 30 ng/mL of 25(OH)D after 12 weeks, which may be justified according to the authors by the small sample size that may have prevented the detection of association between therapy and depletion rates. The authors reported that efavirenz therapy was associated with increased prevalence of vitamin D insufficiency 11. Patients taking efavirenz require higher doses of vitamin D compared to other antiretroviral drugs, since it increases the catabolism of 25(OH)D. However, the precise dose has not yet been defined. A study of HIV-infected patients with HAART 37 demonstrated an increase in bone biomarkers following supplementation with 300,000 IU of vitamin D.
In Longenecker et al. 38 inflammatory biomarkers were not altered by vitamin D supplementation. The statistically significant difference was in the change in IL-6 between groups, with an increase in the probability of the placebo group rather than a decrease in concentrations of IL-6 among those taking vitamin D. However, the authors recommend that further studies are needed to discover the relative importance of inflammatory biomarkers in pathophysiology for the risk of comorbidities in people living with HIV.
Regarding the duration of the intervention, the study by Coelho et al. 23 lasted 24 weeks, in which vitamin D was measured by chemiluminescent assay, and the participants with insufficient vitamin D levels were prescribed a regimen of vitamin D3 supplementation of 50,000 IU, orally, twice weekly for five weeks, followed by another phase with supplementation of 8,000 IU twice a week, for 19 weeks, a regimen that was in agreement with the Society of Endocrinology 33.
The study by Lake et al. 39 was from June 2010 to April 2011, in which vitamin D was also measured by chemiluminescence, and the project participants were divided into two groups: participants with vitamin D insufficiency initiated an oral vitamin D3 supplementation regimen of 50,000 IU twice a week, for five weeks, and then 2,000 IU per day until the end of 12 weeks; and the other group was made up of non-HIV infected people, with low bone mineral density, who underwent a supplementation regimen of 50,000 IU per week, for five weeks, followed by maintenance therapy with 1,400 IU daily.
The five studies aimed to evaluate the effect of cholecalciferol supplementation on vitamin D blood levels and all of them found positive results, in which vitamin D levels were increased after weeks of supplementation, proving the efficiency of supplementation, similar to other studies such as the one by Sheperd et al. 40 and the European AIDS Clinical Society guidelines of 2014, that recommend supplementation in patients with 25(OH)D levels < 10 ng/mL.
Vitamin D supplementation may, therefore, decrease the progression of HIV and prevent the development of tuberculosis and other opportunistic diseases, which makes it more important to carry out clinical studies such as those chosen in this review that help to prove the efficacy of vitamin D supplementation in the prevention of infectious diseases and the very progression of HIV. In patients with vitamin D deficiency, this may be a cost-effective and non-toxic therapeutic option. As vitamin toxicity, characterized by hypercalcemia, is observed very rarely and Hathcook et al. 40 evaluated several studies and did not find any case of intoxication in individuals with daily doses up to 10,000 IU of cholecalciferol.
The protocol of this review was designed considering the recommendations for systematic review and was published allowing the reproducibility of the research and reducing the potential bias of selection of the studies. However, there was a choice of the Portuguese and English languages for the search of the studies and with publication limit of 10 years, which nevertheless ensures a broad scope of the research.
However, this review does not have the limitations, the studies analyzed presented only small samples, not influencing the ability to evaluate the association between changes in 25(OH)D levels and clinical and demographic factors, such as the use of antiretroviral therapy; the majority of the studies do not have national representativeness, and the findings cannot be generalized for women and people of non-white ethnicity; the lack of standardization in the supplementation regime (dose, duration), mainly in the maintenance phase, can influence the final result.
However, the gap remains in the knowledge about the effects of vitamin D supplementation on biochemical markers in people with HIV, which reinforces the need for future research to elucidate these issues. In the meantime, we encourage the adoption of an adequate diet associated with other healthy practices, at the individual level, obviously seeking to find an alternative for the improvement of the vitamin D status of the HIV patient.
CONCLUSION
The results of this systematic review provide evidence that vitamin D supplementation in HIV-positive patients on antiretroviral therapy increased serum levels of 25(OH)D. The five studies reviewed here showed a positive effect of supplementation on lymphocyte counts supporting the vitamin D benefit in immunological recovery, which is particularly relevant for the population living with HIV and effective for a variety of suppressive ART regimens. However, given the scarcity of results, new studies of supplementation with this vitamin in this public are needed to add to the protocol of treatment of the antiretroviral therapy.