INTRODUCTION
Provision of total parenteral nutrition for preterm infants is paramount, especially for very low-weight babies at birth (less than 1.5 kg). Preterm babies often cannot achieve the minimum rate of growth since the requirements of energy expenditure in extrauterine life are higher than those of intrauterine life (1). In addition, preterm babies are often unable to suck or swallow well before 34 weeks of age (2). Therefore, early parenteral nutrition should be added and gradually given along with enteral feeding or breast milk (2,3). In order to get sufficient macronutrients and micronutrients, nutrition should contain dextrose, amino acids, lipids, electrolytes, and vitamins. Currently, ready-to-use parenteral nutrition formulas contain one type of nutrient. However, the administration of each nutrient separately via a different line may become a burden for any hospital. Mixing all those macro- and micro-nutrients in one intravenous (IV) bag will reduce the number of venous lines. Besides, the administration of parenteral nutrition in one bag is also paramount for prematures with volume restriction. Therefore, provision of a high concentrated solution in a low-volume formulation is often preferred. This confirmed why requests for total parenteral nutrition admixtures have currently increased in the hospital pharmacy (4).
Even though the administration of a total parenteral nutrition formula is common practice, the mixing of an all-in-one formulation for administration in one bag remains unusual. Practitioners remain unsure about the stability of added lipids for parenteral nutrition (5). Thus, parenteral nutrition is commonly administered through separate lines, which is then called two-bag parenteral nutrition. However, an issue may arise in the critical care setting, where the patient receives many intravenous medications and venous access is limited (4). The administration of two-bag parenteral nutrition, with a separate lipid route, needs an additional port or access. This situation arouses debate on the benefits of mixing all nutrients into one bag.
The concern when adding lipids into a parenteral nutrition formula is instability, which results in creaming, coalescence, and cracking. The main hazard of macronutrient stability is not a chemical but rather a physical issue associated with particle size distribution. A previous study that assessed total parenteral nutrition in one bag had quite a different result (6 7-8). Additionally, no studies comparing mixed lipid and lipid-free parenteral nutrition using similar formulas for prematurity have been found. This study discusses and compares two types of solution–first, an all-in-one or one-bag parenteral nutrition formula; second, a lipid free or two-bag parenteral nutrition formula.
METHOD
The nutrition components were acquired from standard hospital stock: Aminosteril® Infant 6% (Fresenius Kabi Combiphar), Dextrose 5% (Otsuka), Dextrose 40% (Otsuka), NaCl 3% (Otsuka), Potassium Chloride injection 7,46% (Otsuka), Calcium Gluconate Injection (Generik, Ethica Industri Farmasi), Magnesium Sulfate 20% injection (Otsuka), Lipofudin® 20% (Braun), Nutrient Pad Set Standard TTC Media, Membrane Filter (Satorius), and Pepton Water (OXOID).
FORMULA
This study investigated the standard parenteral nutrition of preterm babies with a weight of 1,000 mg. The composition of nutrients was based on the first-day and second-day guidelines used by practitioners in the hospital (9). A stability study was carried out on four formulas, where formulas 1a and 2a are all-in-one parenteral nutrition (AIO-PN) and formulas 1b and 2b are lipid-free parenteral nutrition (lipid-free-PN) formulas, as stated below:
Formula 1a is a first-day standard formulation in one bag containing 5% glucose (28.85 mL), 40% glucose (25 mL), 6% amino acids (25 mL), 10% calcium gluconate (10 mL), 20% magnesium sulfate (0.36 mL), and 20% lipids (5 mL) (Day-1 AIO PN).
Formula 1b is a first-day standard formulation in one bag containing 5% glucose (28.85 mL), 40% glucose (25 mL), 6% amino acids (25 mL), 10% calcium gluconate (10 mL), and 20% magnesium sulfate (0.36 mL) (Day-1 Lipid free PN).
Formula 2a is a second-day standard formulation in one bag containing 5% glucose (30 mL), 40% glucose (33.33 mL), 6% amino acids (25 mL), 10% calcium gluconate (10 mL), 20% magnesium sulfate (0.36 mL), and 20% lipids (7.5 mL) (Day-2 AIO PN).
Formula 2b is a second-day standard formulation in one bag containing 5% glucose (30 mL), 40% glucose (33.33 mL), 6% amino acids (25 mL), 10% calcium gluconate (10 mL), and 20% magnesium sulfate (0.36 mL) (Day-2 Lipid-free PN).
Four formulas were prepared to be aseptic in triplicate under laminar air flow (LabTech International, Indonesia). After preparation, the four formulas were kept in a refrigerator (2-8 °C). Physical stability was investigated every 24 hours during 7 days following the principle of compatibility justification – visual inspection, particle size, and pH. Each solution was observed by a trained pharmaceutical technician against a black and a white background to detect visual changes including discoloration, effervescence, turbidity, emulsion instability, creaming, and cracking. A particle size analyzer (Horiba, Germany) was used to measure particle size distribution in the sample. The distribution of lipid droplet diameters was also confirmed using a microscope (Olympus CX21, Japan). Chemical detection was evaluated with a calibrated surface pH-meter (Horiba, Germany) and osmometer (Horiba, Germany).
RESULTS
The AIO PN formulas showed that osmolarity was within the 500-700 mOsm range as shown in table I. Based on visual inspection the AIO PN formulas changed visually during testing. Table II shows that creaming developed in AIO PN (F1a and F2a) formulas from the third day after preparation. Particle size was measured as in table III. It was in the range < 600 nm. Table IV shows that the pH of all four formulas did not change significantly during the assay.
Table I. Osmolarity of four parenteral nutrition formulas after preparation
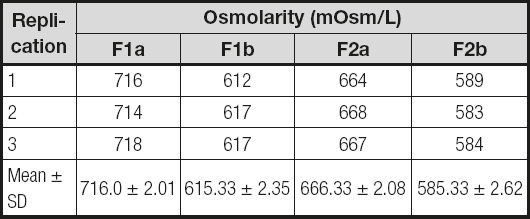
F1a:standard first-day AIO PN formula for preterm babies;
F1b:standard first-day lipid-free PN formula for preterm babies;
F2a:standard second-day AIO PN formula for preterm babies;
F2b:standard second-day lipid-free PN formula for preterm babies.
Table II. Evaluation on physical stability of four formulas based on visual examination

F1a:standard first-day AIO PN formula for preterm babies;
F1b:standard first-day lipid-free PN formula for preterm babies;
F2a:standard second-day AIO PN formula for preterm babies;
F2b:standard second-day lipid-free PN formula for preterm babies.
Table III. Particle size of four parenteral nutrition formulas using a particle size analyzer (PSA)

F1a:standard first-day AIO PN formula for preterm babies;
F1b:standard first-day lipid-free PN formula for preterm babies;
F2a:standard second-day AIO PN formula for preterm babies;
F2b:standard second-day lipid-free PN formula for preterm babies.
Table IV. pH value of four parenteral nutrition formulas using a pH-meter

F1a: standard first-day AIO PN formula for preterm babies; F1b: standard first-day lipid-free PN formula for preterm babies; F2a: standard second-day AIO PN formula for preterm babies; F2b: standard second-day lipid-free PN formula for preterm babies
DISCUSSION
The osmolarity value, which is higher than 600, may be categorized as hyperosmolar. It showed that these four standard formulas should not be administered through peripheral routes, to prevent vein problems such as phlebitis or extravasation. A central route or PICC is best for these formulas, especially in babies or young children. Of all four options, AIO PN formulas had a higher osmolarity around 80-100 mOsm as compared to the Lipid-free PN ones.
Emulsion instability is marked by creaming, cracking, and inversion phase. Cracking is due to particle fusion to form larger particles. Creaming indicates physical instability and is reversible with shaking. Irreversible instability occurs when coalescence or cracking develops. During seven days in observation no visual signs of coalescence or cracking appeared. Coalescence and cracking typically occur when fat globules are larger than 500 nm (10). As regards the particle size analysis, the four formulas remained within the normal range (300-400 nm) for seven days, though physical changes were seen. To better qualify results we chose PSA rather than visual inspection. PSA measured the particles following a principle of dynamic light scattering, which is more sensitive and specific compared to the eye ability to differentiate emulsion changes. PSA is able to detect particles accurately in a range of 0.01-5,000 µm, and is also reliable to measure lipid size distribution. Furthermore, the microscopic analysis revealed no particles larger than 1 µm, and the naked eye is limited to sizes larger than 50 microns (5). Therefore, it was confirmed that judgment of lipid physical instability within 24 hours as based on naked eye evaluation is inaccurate.
Evidence of particulate hazards has been reported, such as deaths in newbors associated with embolism. Therefore, such formulations should be avoided for parenteral or intravascular administration (11). Table II shows that all four formulas remain within the acceptable range. The particle sizes obtained are able to freely circulate through the microvascular network. Particle sizes larger than the microvascular diameter (600 nm-1,000 nm) are dangerous since particles would be trapped inside these veins and induce embolism (12,13). Any particles larger than the vasculature’s diameter may block a vein or artery. They also may occlude the capillary bed since the particles be larger than capillary diameter. During intravenous delivery, particulate contaminants enter the vein and travel through the venous system to the heart and lung. Particles larger than 5 µm tend to become trapped in the lung, whereas those smaller than 5 µm are usually retained in the liver, spleen, or kidney (14). In order to achieve a safe particle size, intravenous medication or parenteral nutrition particles should not be larger than 1 µm (15). Particle sizes smaller than 600 nm are physically safe to be introduced and circulate in the blood vessels.
Furthermore, the results of pH-metry show that the addition of fat into a parenteral nutrition formula causes a pH decrease (1.06 units for the first formula, 1.24 units for the second formula), even though the pH of the four formulas, including the ones for AIO PN, did not change significantly during seven days in a refrigerator. In theory, any lipids (6 7-8,11) added to glucose as an acid compound will reduce the lipid pH but increase the PN pH. However, the value of the final PN pH, around 6, is commonly stable; instability may begin when the PN pH is lower than 5.
AIO PN stability was influenced by composition, concentration, and environment, as well as by storage conditions. This study confirmed the causes of physical stability in AIO PN formulas. Both AIO and lipid-free parenteral nutrition formulas remained physically stable to visual inspection, pH-metry, and particle size analysis. Although the formula developed creaming, this was easy to disperse after soft shaking. Hence, it was considered to be safe, without no large globules. This result differs from that of a previous study that used different lipid sources and formulas (16). That study identified AIO PN instability within minutes (16). In addition, previous studies did not add vitamin to the parenteral nutrition formula. Vitamins such as Vitalipid® act as fat-soluble vitamins with antioxidant activity to prevent peroxide formation, but do not influence particle size. Therefore, they may enhance stability. Meanwhile, this current research is similar to the previous study that stated AIO PN was stable when fat-soluble vitamins were added (17), with stability persisting for up to seven days (6). The provision of AIO parenteral nutrition in one single bag will be beneficial for patients; it will also be cheaper, with fewer venous accesses required, and simpler to administrate.
This study assessed physical changes such as particle size, as larger sizes indicate physical instability, threaten the microvascular tree, and may result in death. However, it has not solved the chemical stability issue, which is related to concentrations.














