INTRODUCTION
Non-cystic fibrosis bronchiectasis (NCFB) is a chronic disease characterized by permanently dilated airways due to bronchial wall structural component destruction as a result of a vicious cycle involving persistent bacterial colonization and chronic neutrophilic infiltration of the bronchial mucosa (1). Bronchiectasis causes pulmonary infections and loss of lung function, which results in chronic morbidity contributing to premature mortality (1).
Systemic inflammation as demonstrated by increased blood neutrophil levels, elevation of C-reactive protein (CRP), and plasma cytokines has been reported in patients with NCFB, correlating also to disease severity and bacterial colonization (2,3). This systemic inflammation has also been suggested as a cornerstone of reduced exercise capacity and peripheral muscle strength in patients with bronchiectasis (4). Increased blood neutrophil levels have been associated with NFCB disease severity and bacterial colonization in NFCB patients, being considered a possible marker of systemic inflammation (3). Intense oxidant and anti-oxidant activity is also present in the airways and circulating blood of patients with bronchiectasis (5). Overproduction of reactive oxygen species (ROS) results in oxidative stress that can lead to inflammation. This is thought to be an important factor in the pathogenesis and progression of bronchiectasis (5,6).
Enhancing effective expectoration of stagnated bronchopulmonary secretions is essential for the clinical management of bronchiectasis. Exercise training or a pulmonary rehabilitation program (PR) has beneficial effects on NCFB patients (7), and is recommended in current NCFB treatment guidelines (8,9). These effects are related to the respiratory and peripheral skeletal muscle manifestations of bronchiectasis and are commonly associated with improvements in respiratory symptoms, including mucus removal (7,10). Despite the relevant role that inflammation is believed to have in the progression of bronchiectasis (3,4), and its relationship with oxidative stress status (5,6), to the best of our knowledge there are no studies evaluating whether exercise is able to modify the inflammatory and oxidative response of patients with NFCB; only unclear results have been reported in chronic obstructive pulmonary disease (COPD) patients (11, 12, 13).
Beta-hydroxy-beta-methylbutyrate (HMB) is a derivative, in vivo, of the essential amino acid leucine (14) with a well-documented anti-catabolic effect on skeletal muscle in both healthy and pathological conditions (15, 16 17). Our group has already published the positive effects of the addition of a hyperproteic oral nutritional supplement enriched with HMB together with a PR program on body composition, BMD, muscle strength, health-related quality of life, and breathing parameters in patients with bronchiectasis (16, 17 18). But HMB supplementation has been also suggested as exerting positive anti-inflammatory effects (19), which might be associated with improved pulmonary function in COPD patients (20). It has been suggested that some pro-inflammatory factors, such as interleukin 1 beta and tumor necrosis factor alpha, increase proteolysis and may modulate protein turnover (21). Because HMB is associated with less proteolysis (22), to date, several studies have suggested that HMB supplementation could affect inflammatory responses even after exercise (19).
The aims of this study were to evaluate in untrained NCFB patients the effects of a PR program, alone or combined with the intake of a hyperproteic oral nutritional supplement enriched with HMB, on oxide-reduction (REDOX) and inflammatory biomarkers.
MATERIALS AND METHODS
Study design, inclusion/exclusion criteria, and screening process are as previously described (16, 17, 18). Briefly, a total of 30 stable (without respiratory exacerbations) patients were recruited from the bronchiectasis unit at the Regional University Hospital of Málaga from 2013 to 2014, and randomized in a 1:1 ratio to undergo either PR alone or PR with nutritional support (PRS). Inclusion criteria were as follows: a diagnosis of NCFB, age 18 to 80 years, normo-nourished, and without any acute disease.
INTERVENTION
The exercise program consisted of 60 minutes of exercise biweekly at the hospital, and one unsupervised session per week for twelve weeks (16). Hospital and home exercise sessions consisted of a total of 45 min of exercises, coupled with 15 min of breathing retraining with the Orygen-Dual Valve®, and 7-10 minutes of stretching and relaxation exercises. Attendance of the sessions at hospital was recorded and at-home sessions were reminded by telephone every two weeks. Physical activity was evaluated using the wGT3X (ActiGraph) accelerometer and the IPAQ (International Physical Activity Questionnaire) (23), with no differences in physical activity level according to supplement intake status (18).
The supplement intervention (16) was based on the intake or not of the oral nutritional supplement Ensure Plus Advance, which is a 220-mL, oral hyperproteic nutritional supplement providing 330 kcal (1.5 kcal/mL), 18 g of protein, 1.5 g of HMB and 1.7 g of prebiotic fiber (FOS). The participants were advised to take the supplement at least 60 minutes before the rehabilitation sessions. As all were normally nourished, a registered dietitian informed them of how to reduce the intake of natural foods in order to compensate for the increase in calories supplied. Adherence was recorded by patients in a diary and controlled by the investigators in the PR sessions or by telephone. During the intervention, all participants were instructed on general recommendations for a Mediterranean-style healthy diet.
A seven-day, prospective dietary questionnaire was fulfilled by all participants in their homes. The dietary data was registered into their personal computer equipped with the software package Dietstat (Dietstat® 1.0, 2010, Málaga, Spain) with up-to-date composition tables for Spanish food (24). Macronutrient intake did not show significant differences according to supplement intake status at any time points (16).
Once the PR finished, participants were instructed and subsequently reminded to maintain a non-sedentary physical activity level and to use the Orygen-Dual Valve® (25) twice weekly, as well as to maintain a Mediterranean-style healthy diet during the follow-up period.
The study was approved by the Málaga Provincial Research Ethics Committee. All the procedures were conducted according to the Declaration of Helsinki, and all of the participants provided their written informed consent. The study was registered at the clinicaltrials.gov site (http://clinicaltrials.gov) NCT02048397.
OUTCOME MEASURES
Outcome assessments were performed at baseline, 3 months and 6 months.
Once no respiratory exacerbations were confirmed, a serum sample was taken and separated: one aliquot was immediately stored at -80 °C and another aliquot was immediately used to measure the biochemical parameters. The SSPA Biobank has coordinated the -80 °C storage of the serum samples.
Weight and height were measured and BMI was calculated.
Circulating levels of interleukine 6 (IL-6), tumor necrosis factor alpha (TNFα), and adiponectin were measured by enzyme immunoassay (EIA) (R&D Systems Europe Ltd). Neutrophil and CRP circulating levels were determined by the Central Assistance Laboratory of Hospital Regional de Málaga. Also, serum oxidative markers 8-isoprostane (Cayman Chemical, Michigan, USA), total antioxidant capacity (TAC) (Cayman Chemical, Michigan, USA), and superoxide dismutase activity (SOD) (Cayman Chemical, Michigan, USA) were determined by EIA. The circulating levels of thiobarbituric acid reactive substances (TBARs) were determined by spectrophotometry (26).
Fold change was calculated as the change described by the ratio between the levels of circulating metabolites at 6 months of study and baseline levels. They were expressed as percentages.
STATISTICAL METHODS
Sample size was calculated based on the circulating levels of 8-isoprostane in bronchiectasis patients previously reported by our group (5). For an 80 % probability of detecting a difference in 8-isoprostane levels after months of intervention a total of 30 participants were required, based on the assumption of a difference between groups of 33 pg/mL, with a standard deviation (SD) of 31.9 pg/mL.
Analyses were performed using the SPSS statistical software. Descriptive results are shown as mean and standard deviation. The Shapiro-Wilk test was used to assess whether the variables were normally distributed. Hypothesis contrast for continuous variables between groups used Student’s t-test for variables that followed a normal distribution and non-parametric tests for variables that did not conform to normal. Variables tested repeatedly over time following a normal distribution were analyzed using repeated measures multiple analysis of variance. The effects of time on the variables tested repeatedly over time that did not follow a normal distribution were analyzed by a Wilcoxon test within samples under the same treatment. All analyses were performed using the SPSS statistical software, version 20.0. NY (27).
RESULTS
A total of 30 individuals (15 in each group) were enrolled in the clinical trial. All participants completed the 3-month PR program. Two participants from each arm withdrew from the study during the 6-month follow-up period because of an illness unrelated to bronchiectasis (16, 17, 18).
The clinical baseline characteristics, summarized in table I, and the evolution of the dynamometry and quality of life parameters, as well as serum or plasma biomarker levels, of the participants have been previously described (16). The number of exacerbations suffered by the patients during the previous year and one year after commencing the intervention was similar for both groups (17). Additionally, no differences in weight, height were found according to supplement intake status at any time point (data not shown).
Table I. General clinical characteristics

Data are average ± standard deviation. PR: pulmonary rehabilitation; PRS: pulmonary rehabilitation plus oral nutritional supplement; BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in one second.
*Statistical differences comparing PR vs. PRS.
NEUTROPHIL LEVELS
There were no differences in white blood cell levels throughout the study period except for neutrophil levels. The PRS group showed significantly lower neutrophil levels at 6 months than at baseline (neutrophils at baseline (x 109/L): 4.24 ± 1.55 vs. neutrophils at 6 months (x 109/L): 3.62 ± 1.09, p = 0.01).
Neutrophil levels were the only blood cells that showed statistically different fold-change percentages according to supplement intake status, being positive in the PR group but negative in the PRS group of patients (Fig. 1).
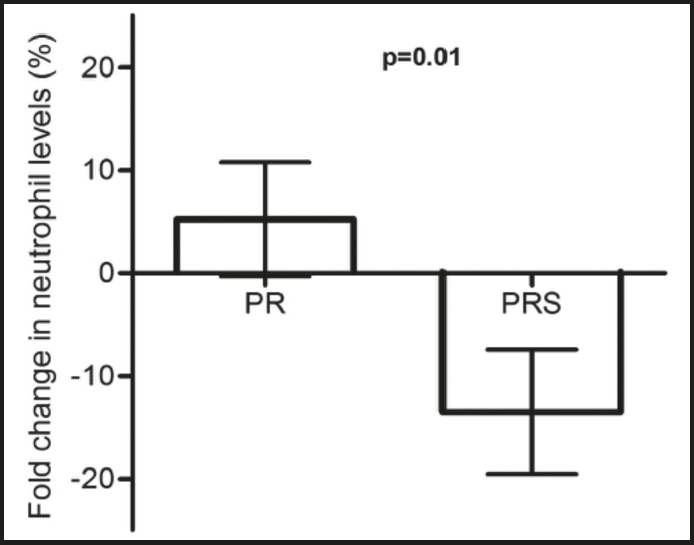
Figure 1. Fold-change percentage in neutrophil levels. Bars represent the mean and standard deviation values of the fold change percentage (month 6 - baseline) in neutrophil levels of NCFB patients following a pulmonary rehabilitation (PR) program, and NCFC patients following the same pulmonary rehabilitation program supplemented with HMB (PRS).
INFLAMMATORY BIOMARKERS
In table I it is shown the evolution of the evaluated inflammatory biomarkers throughout the study. CRP levels keep stable during the 6 months with a trend to decrease their circulating levels (Table II). The other studied biomarkers showed a different behavior over time; thus, TNFα levels presented an initial decrease at 3 months, significantly different from the baseline level when considering the total group of patients and the PR group, which remained significantly lower by the end of the study in the PR group (Table II), while IL-6 levels tended to rise during all the study period, with IL-6 levels at 6 months being significantly higher than at 3 months in the total group of patients and the PR group (Table II). Alternatively, adiponectin levels were significantly higher from baseline at the end of the study in the total group of patients and the PR group, but not in the PRS group (Table II).
Table II. Inflammatory biomarker evolution throughout the study period
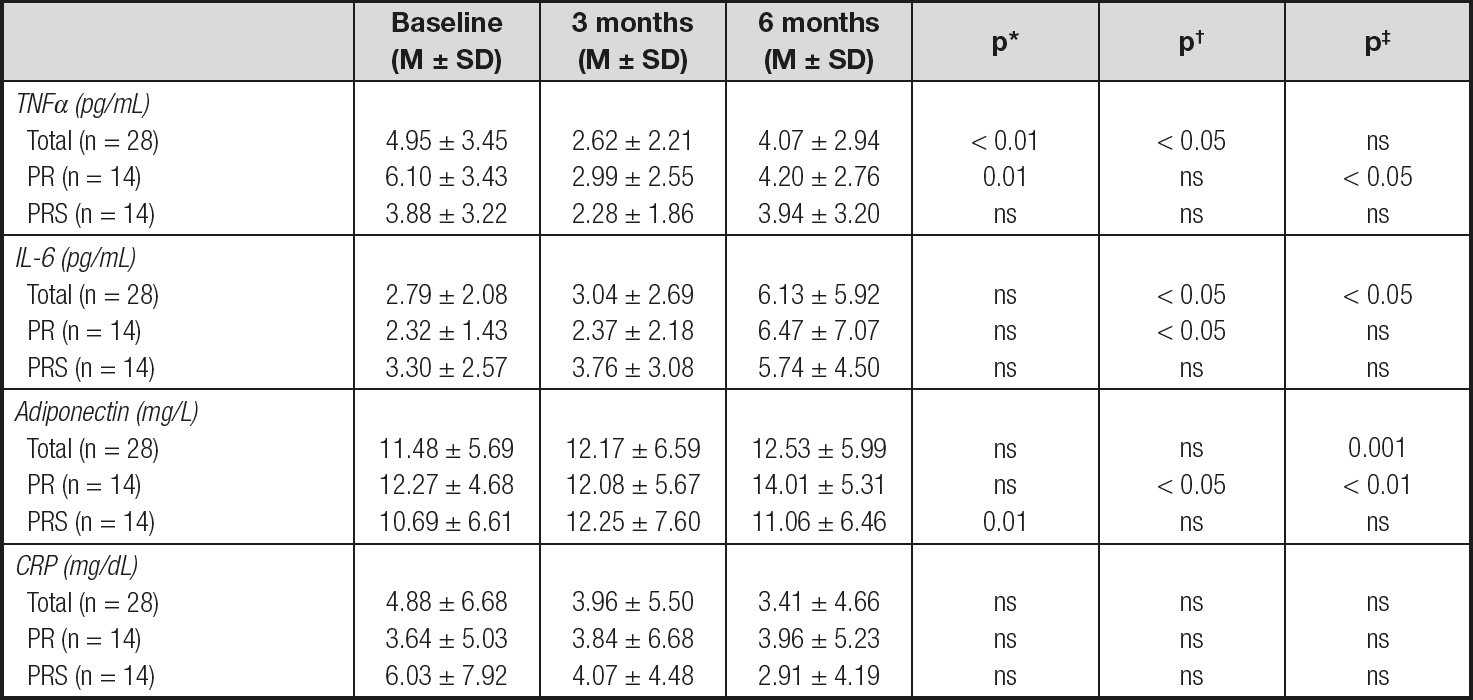
M ± SD: average ± standard deviation; TNFα: tumor necrosis factor alfa; IL-6: interleukin 6; CRP: C-reactive protein; PR: pulmonary rehabilitation; PRS: pulmonary rehabilitation plus oral nutritional supplement.
*Statistical differences comparing baseline vs. 3 months.
†Statistical differences comparing 3 months vs. 6 months.
‡Statistical differences comparing baseline vs. 6 months
None of the studied biomarkers of inflammatory status or their fold-change percentages were different according to treatment.
REDOX BIOMARKERS
The circulating levels of the studied REDOX biomarkers remained stable throughout the study except for 8-isoprostane levels, which were increased to produce significantly higher levels from baseline after 6 months of treatment in all group of patients (Table III). Moreover, in the total and PRS groups, 8-isoprostane levels at 6 months were also significantly higher than at 3 months.
Table III. Oxidative biomarker evolution throughout the study period
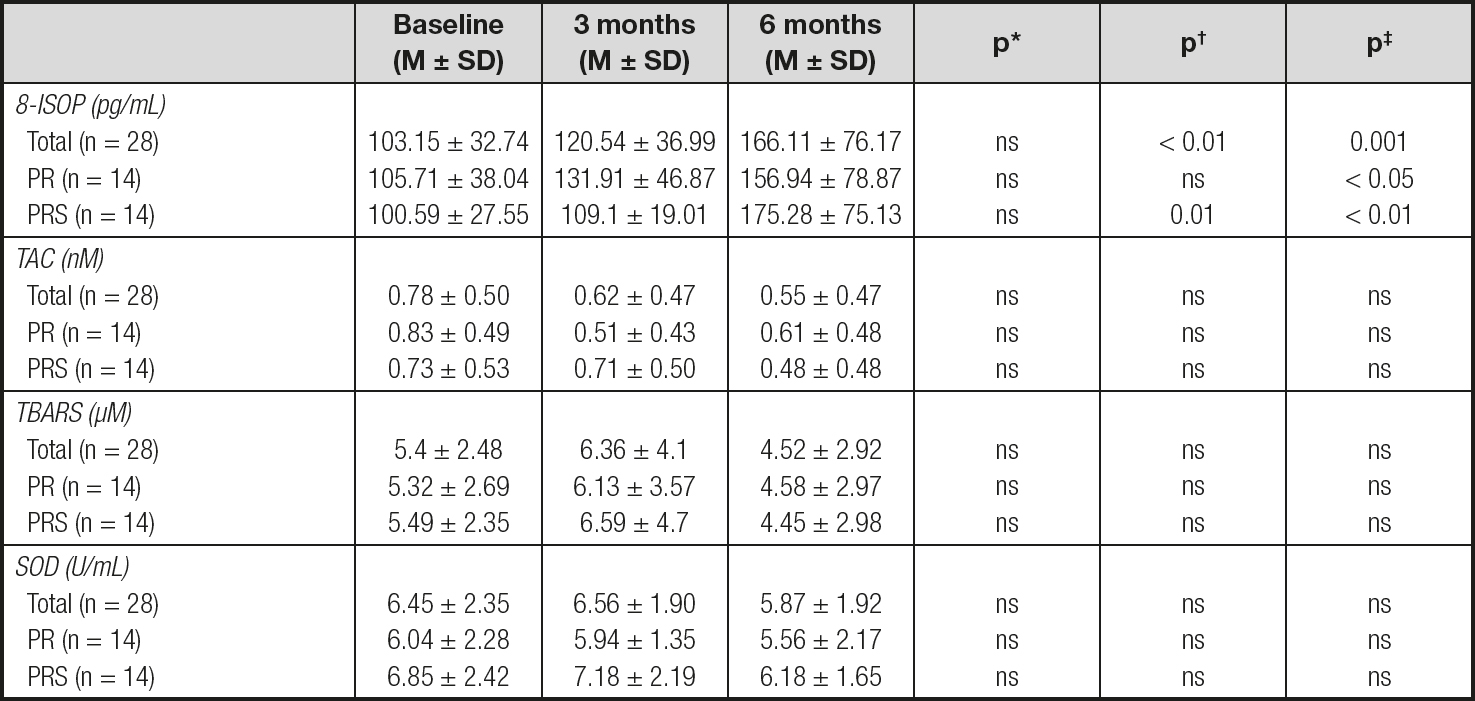
M ± SD: average ± standard deviation; 8-ISOP: 8-isoprostane; TAC: total antioxidant capacity; TBARS: thiobarbituric acid reactive substances; SOD: superoxide dismutase; PR: pulmonary rehabilitation; PRS: pulmonary rehabilitation plus oral nutritional supplement..
*Statistical differences comparing baseline vs. 3 months.
†Statistical differences comparing 3 months vs. 6 months.
‡Statistical differences comparing baseline vs. 6 months
Neither REDOX biomarker levels nor their fold-change percentages were significantly different between the PR and PRS groups.
DISCUSSION
This study revealed that PR could exert a pro-oxidative effect accompanied by changes in circulating inflammatory cytokine levels in normally nourished bronchiectasis patients. Furthermore, the addition of a hyperproteic oral nutritional supplement enriched with BHT to PR may reduce neutrophil levels when compared to PR alone. These assessments are important because studies suggest that oxidative stress and inflammation are strongly related to and involved in the pathogenesis and progression of bronchiectasis disease (1,5,6). Accordingly, the information provided by this study could be useful for choosing the right therapeutic approach in the management of bronchiectasis disease.
It has been described that inflammatory and oxidative responses in NFCB are mainly caused by chronic neutrophilic activation (1,5,6), this having been considered a potential marker of systemic inflammation (3). The impact of exercise on neutrophil levels is unclear. It has been documented that highly intensive exercise elicits mobilization and functional augmentation of neutrophils in healthy sedentary adults (28,29); however, in adolescents with obesity the number of neutrophils is both reduced and increased after 6 months of high- and low-intensity training, respectively, when compared with baseline (30). To the best of our knowledge, no previous studies have reported information about the regulation of neutrophil levels by exercise in bronchiectasis. In this study, exercise alone did not have any effect on neutrophil levels over time; however, the intake of the nutritional HMB-enriched supplement led to a reduction in neutrophil circulating levels at the end of the study when compared with baseline values.
A different fold-change percentage in neutrophil levels was found between the PR and PRS groups of patients. It is known that HMB is able to modify immune cell function in human (31) and non-human models (32). Similar to the present study, supplementation with HMB in COPD patients was associated with a reduced number of total leukocytes when compared with a control group without supplementation (20). All in all, our results might be suggesting a beneficial effect of HMB on the regulation of systemic inflammation in NFCB patients through a reduction in neutrophil levels; nevertheless, this result should be taken cautiously due to the lack of data about chronic neutrophil activation in the present investigation.
Several investigations of systemic inflammatory markers in COPD patients after an exercise program with and without nutritional supplementation have already been done. However, no information in this regard is available for NFCB patients.
It is not clear whether exercise alone may modify inflammatory response in individuals with COPD, with investigations reporting different results (11,13,28,33). In our study, exercise alone was able to reduce TNFα and increase adiponectin levels at the end of the study, and there were also close-to-significant increases in circulating IL-6 levels. The lack of significance in the increased IL-6 levels found in the PR group was probably due to an insufficient sample size. According to our results, the inflammatory response to a PR program in NFCB patients is unclear, with both an anti-inflammatory effect on TNFα levels and a pro-inflammatory effect on IL-6 and adiponectin levels (34). Contrary to these observations, in the PRS group of patients inflammatory biomarker levels remained stable throughout the study period. All this might be pointing to a balancing effect of HMB on the inflammatory changes caused by exercise in untrained NFCB patients.
There are few works evaluating the effect of HMB on inflammatory biomarkers, and most of them are focused on athletes (35,36); in all of them HMB seemed to attenuate the inflammatory response to intense exercise with a reduction in cytokine production. Studies in COPD patients comparing the effect on inflammatory response of the intake or not of a nutritional supplement added to a PR program returned different results according to supplement composition (37,38). In a study comparing the systemic inflammatory effect of a nutritional supplement based on whey peptide, ω-3 fatty acids and antioxidant vitamins, compared with a control group without the nutritional supplement, in COPD patients, the intake of the supplement decreased CRP, IL-6, and TNFα levels, among others (38). Contrary to this, in our study, the levels of inflammatory biomarkers were no different according to supplement intake status. Two main reasons might be explaining the differences with our study: first, the nutritional composition of each supplement was different. Secondly, the COPD patients of the Sugawara et al. study were malnourished whereas our NFCB patients were normally nourished.
Considering that higher inflammatory cytokine levels are associated with higher muscle and weight loss (39), together with the fact that COPD undernourished patients show higher levels of inflammatory cytokines (40,41), nourished status might be affecting the anti-inflammatory effect of the above supplements.
To the best of our knowledge there are no previous studies evaluating the effects of a PR program on the oxidative biomarkers of NFCB patients; however, some of these effects have been described in COPD patients with contradictory results according to disease severity (12,42) or exercise intensity (42, 43 44). Some studies have reported an increment in lipid peroxidation and protein oxidation, accompanied by a decrease in antioxidant mechanisms, in muscle biopsy samples in response to high intensive exercise training in both stable (43) and severely unstable (12) COPD patients, whereas some other authors have reported no differences in muscle lipid peroxidation (44) after high-intensity exercise, or even an improvement of muscle oxidative stress in severe COPD patients (42). In line with those studies reporting a pro-oxidative effect of exercise, in our study an increment in 8-isoprostane levels was observed in response to exercise, regardless of supplement intake status. The fact that no other oxidative biomarker showed differences after the exercise program might be explained because we evaluated systemic oxidative biomarker levels instead of changes in muscle tissue, where the production of ROS is higher (45). Nevertheless, it has been described that in biological fluids the levels of 8-isoprostane seem to represent an accurate measure for in vivo oxidative status, and they have been considered the gold-standard biomarker for lipid peroxidation (46). Among the possible physiological functions proposed for 8-isoprostane, some authors have suggested that it could be involved in the regulation of the exercise response (46). Apart from the deleterious effects of muscle ROS increments in response to non-regular intensive exercise, some previous investigations pointed to a possible positive effect of increased ROS production after regular physical training, which would lead to activate the exercise-induced adaptation of the muscle phenotype (47). In all, this led the authors to suggest a systemic pro-oxidative effect of the PR program that might be associated with a possible exercise-induced adaptation.
In our study, the group of NFCB patients nutritionally supplemented showed a similar increment in 8-isoprostane levels as that of the patients not supplemented, without differences in any other oxidative biomarkers. According to these, the nutritional support enriched with HMB during a PR program did not modify the pro-oxidative effect observed after the exercise in untrained NFCB patients.
Some limitations have been identified in this study. Studies of neutrophil function would be interesting in order to interpret our results; however, no fresh samples for neutrophil function or activation studies were taken during the sample collection.
Additionally, airway samples, in which levels of inflammatory markers could be determined as a more sensible system to detect changes, were not possible to obtain for this study; nevertheless, several studies have proposed systemic inflammatory markers as representative of the inflammatory status of NFCB patients, also correlated to disease severity and bacterial colonization (2,3). It has been described in COPD patients that inflammatory and oxidative status are closely related with nourishment state (40,41) and disease severity (13), so the fact that our NFCB patients showed moderate-to-severe breathing involvement without being malnourished could undervalue our results. In the same way, exercise intensity (44,45) and supplement dose (48) have been associated with differences in the inflammatory or oxidative response of COPD patients. Finally, although the sample size was calculated based on the 8-isoprostane circulating levels of NFCB patients (5), it is possible that this sample size was not enough to detect differences in other markers. All of these factors could be considered limitations that should be solved in future investigations in order to complement our results.
In conclusion, the results of our study provide insights about a pro-oxidative effect of a pulmonary rehabilitation program on untrained NFCB patients, accompanied by changes in circulating inflammatory cytokine levels. Additionally, a possible beneficial effect on the reduction of neutrophil levels, a systemic inflammatory biomarker, is observed after the intake of a hyperproteic HMB-enriched supplement on these patients. However, as neutrophil function or chronic activation has not been determined in this study, and no other inflammatory biomarkers were different between the PR and PRS groups, the possible beneficial effects of HMB on the inflammatory status of these patients should be interpreted cautiously. The authors suggest the necessity of further investigations to confirm the possible benefits of HMB on neutrophil activation and function, and also to evaluate the effects of nutritional supplements in NFCB patients with different grades of malnutrition.














