INTRODUCTION
Overweight is a public health issue related to non-communicable diseases (NCDs) both in developed and developing countries (1). This is due to the fact that obesity is a risk factor for the emergence of diseases, including hypertension, type-2 diabetes, and cardiovascular diseases. Therefore, the obesity epidemic must be the main focus of health policies, programs and actions. Thus, it is essential to implement preventive strategies such as early identification of individuals at higher risk, which may generate lower healthcare expenditures (2).
One of the strategies for early diagnosis is performing a body composition assessment, enabling the detection of potential risk to health. This assessment contributes to identify body fat accumulation even in individuals with an appropriate body weight (3). Another harmful factor related to weight gain is increased visceral adipose tissue (VAT) (4).
Increased body fat is related to the expression of the adipokines tumor necrosis factor (TNF-α) and interleukin 6 (IL-6), which contribute to the emergence of diseases (5). However, it is not always possible to measure cytokines in professional practice. One of the strategies that can be applied to assess body fat accumulation is an anthropometric assessment. This is stated based on researches (6 7-8) that show an association between anthropometric measures, VAT, and risk of diseases, but additive and interactive effects with visceral adiposity based on the use of cytokine indexes are still poorly studied.
A risk group for changes in VAT are undergraduate students, since they are predisposed to metabolic changes resulting from excessive visceral and body fat. This can be justified by lifestyle changes driven by joining the university, which may instigate low levels of physical activity and higher consumption of calorie-rich fast-food and ultra-processed foods (9 10 11-12). Among students, women constitute a group that physiologically may have higher fat mass and lower fat-free mass, as men have higher body weight and waist circumference values (13). These are key features to develop disease risk factors (7). These changes, triggered by excessive body fat, also increase circulating cytokines such as IL-6, C-reactive protein (CRP) and TNF-α (14), which promote the occurrence of atherosclerosis, hypertension, insulin resistance, dyslipidemia, and lipid profile changes (15,16).
Therefore, this paper aims to identify these cytokines and associate them with distinct indexes of total and central body adiposity in young female undergraduate students.
METHODS
SAMPLE
The current research is the baseline study for the Project “Efeito do exercício físico no controle metabólico, marcadores inflamatórios, adipocinas e microbiota intestinal” (Physical activity effects on metabolic control, inflammatory markers, adipokines, and gut microbiota). This project was carried out with a sample that consisted of 75 volunteer female sophomore students, aged 18 to 25 years, from a Brazilian public university.
In order to assess the selection criteria, we evaluated sedentary or insufficiently active subjects (17) with “regular” menstrual cycles and without impairments (physical, intellectual, visual, hearing). Pregnant women, mothers with children up to 6 months of age, pacemaker users, and women taking psychotropic medication, undergoing nutritional follow-up, or suffering from diabetes or hypertension were excluded.
The sample frame of the current study consisted of 58 female undergraduate students who had available data related to cytokines.
ETHICAL PROCEDURES
The current study was approved by the Ethics Committee at Universidade Federal de Viçosa – UFV, protocol number CAAE: 53452916.3.0000.5153. All procedures were carried out according to the Guidelines Regulating Research Involving HumanBeings (Resolution 466/2012 of the National Health Council). All the individuals participating in it signed an informed consent form in accordance with the Declaration of Helsinki from 1975, revised in 1983.
ANTHROPOMETRIC INDEXES
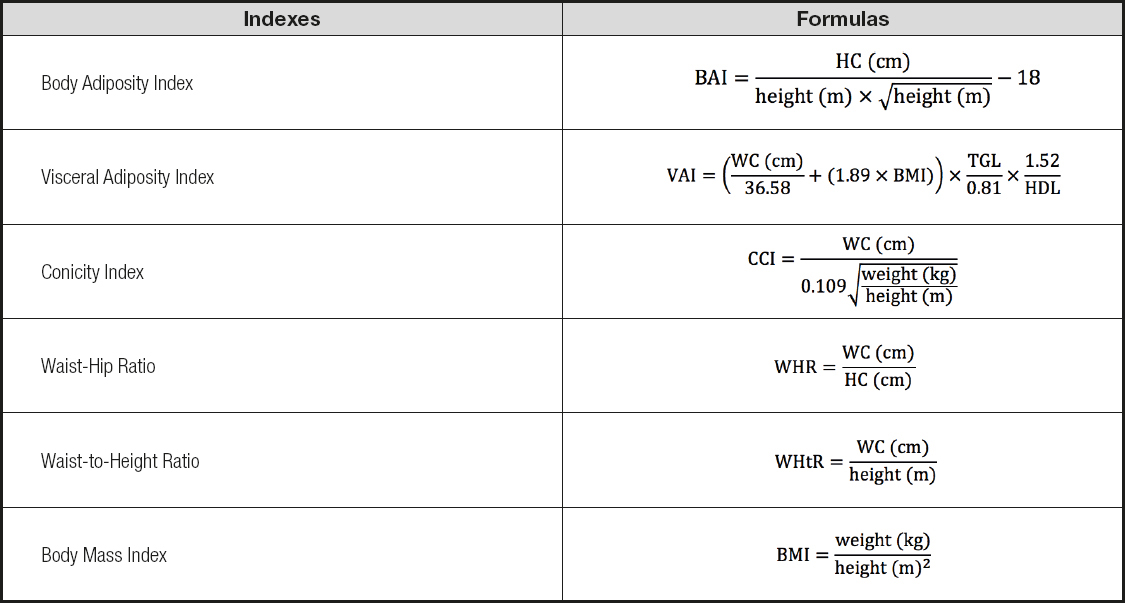
Body weight was measured in kilograms by deploying a Kratos-cas® scale. Height was measured in centimeters using a vertical portable stadiometer (Alturexata®, Belo Horizonte, Brazil). Waist circumference (over the umbilical scar and at the midpoint between the lowest rib and the iliac crest) and hip circumference were measured using a flexible non-elastic measuring tape following the International Society for the Advancement of Kinanthropometry (ISAK) recommendations. Body adiposity index (BAI) (18), visceral adiposity index (VAI) (8), conicity index (CCI) (19), waist-hip index (WHR) (20), waist-to-height ratio (WHtR) (21) and body mass index (BMI) (20) were calculated (Table I).
BODY COMPOSITION
Body composition was assessed from 7 a.m. to 9:30 a.m. using dual energy X-ray absorptiometry (DXA) (Lunar Prodigy Advance DXA System®– analysis version: 13.31, GE Healthcare, Madison, WI, USA). Data regarding total body fat (TBF), regional fat (android and gynoid), lean body mass, and free fat mass were obtained. The subjects fasted for 12 hours and followed the DXA manufacturer recommendations before performing this procedure.
The android region was measured around the waist, between the midpoints of the lumbar spine and the upper part of the pelvis, whereas the gynoid region was measured approximately between the femoral head and mid-thigh (hip). Before each scan session, the device was calibrated according to the pattern procedures recommended by the manufacturer.
The DXA data obtained were used to calculate the fat mass distribution index 1 (FMI1), mass distribution index 2 (FMI2), and android-gynoid ratio (AGR) (Table II).
Table II. Adiposity indexes, calculated from DXA results, and their formulas

FMI1: fat mass distribution index 1; FT: percentage of fat in the torso; FL: percentage of fat in the legs; FMI2: fat mass distribution index 2; FMT: fat mass in the torso; FML: fat mass in the limbs (fat mass in arms and legs); AGR: android-gynoid ratio; AF: android fat; GF: gynoid fat.
The undergraduate students who presented AGR results ≥ 1 were identified as android, while the ones with AGR results < 1 were identified as gynoid.
INFLAMMATORY CYTOKINES, CHOLESTEROL AND FRACTION
Blood samples were collected from 7 a.m. to 9 a.m., after a 12- hour fasting period, by trained professionals in an accredited laboratory. They collected 4 mL of blood from the cubital vein, which were later centrifuged in order to separate the serum from the other blood components. Total cholesterol, low density lipoproteins (LDL), high density lipoproteins (HDL) and triglycerides (TGL) were measured.
Plasma was stored at -80 ºC. Then, cytokines IL-8, IL-1β, IL-6, IL-10 and TNF-α were measured using the Cytometric Bead Array (CBA) Kit for inflammatory cytokines (INFLAMMATORY CYTOKINES CBA KIT, BD, Pharmingen, USA; Catalog no. 551811). The CBA Kit merges together the technologies of ELISA and flow cytometry, which uses polystyrene balls labeled with fluorescence at several levels (22). The data obtained from flow cytometry (FACScalibur, BD, USA) were analyzed using a specific software for CBA (FCAP Array TM Software, BD, Pharmingen, USA) through calibration curves resulting from the kit's cytokine patterns. After that, the concentration of analytes in the sample was determined in pg/mL.
STATISTICAL ANALYSES
The statistical analyses were carried out with the statistical software Stata (version 13). Furthermore, a Shapiro-Wilk test was applied to check the normality of quantitative data. Their descriptive analysis is presented as median and interquartile interval.
Spearman's correlation coefficients were applied to check the correlation between the indexes of central and body adiposity (BMI, BAI, VAI, CCI, WHtR, WC, WHR, AGR, FMI1 and FMI2) and the study cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12 and TNF-α).
In a bivariate analysis, the regression coefficient and the confidence interval (CI) were estimated through linear regression, with adjusted variables at p ≤ 0.25 for inclusion in the model. The level of significance applied was α = 5 %. The White test was applied to identify heteroscedasticity in the distribution of errors in order to verify the adequacy of the linear regression model.
RESULTS
Fifty-eight female undergraduate students aged 18 to 25 years (± 1.82) were assessed. According to the body composition assessment, the average percentage (%) of total fat resulting from DXA was 33.61 % (± 0.94), whereas the ratio percentage of android fat to gynoid fat was 0.61 % (± 0.02). These data show that body fat is located mostly in the hip area (gluteofemoral region and thighs) (Table III).
Table III. Physical and metabolic features of young female undergraduate students

DXA: dual-energy X-ray absorptiometry; BMI: body mass index; WHR: waist-hip ratio; WHtR: waist-to-height ratio; LDL: low density lipoproteins; HDL: high density lipoproteins; TGL: triglycerides; IL: interleukin; TNF-α: tumor necrosis factor.
It was seen that all adiposity indexes, except VAI, were correlated with cytokines, which were identified as weak. It is important to highlight that IL-1β did not correlate with any of the indexes, while TNF-α correlated with 70 % of them (Table IV).
Table IV. Correlation between interleukins and total and central adiposity indices in young female undergraduate students

BMI: body mass index; BAI: body adiposity index; VAI: vascular adiposity index; CCI: conicity index; WHtR: waist-to-height ratio; WC: waist circumference measured over the umbilical scar; WHR: waist-hip ratio; AGR: android-gynoid ratio; FMI1: fat mass distribution index 1; FMI2: fat mass distribution index 2; IL: interleukin; TNF-α: tumor necrosis factor.
Table V presents the final models of the multiple linear regression analysis; increased interleukins relate to the increased indexes CCI, WHR, FMI1 and FMI2, regardless of age and family history of obesity. The other indexes did not meet the assumptions of the linear regression, that is, the homoscedasticity and normality of the residuals.
Table V. Final models of the multiple linear regression analysis between body adiposity indexes (dependent variables) and cytokines in young female undergraduate students
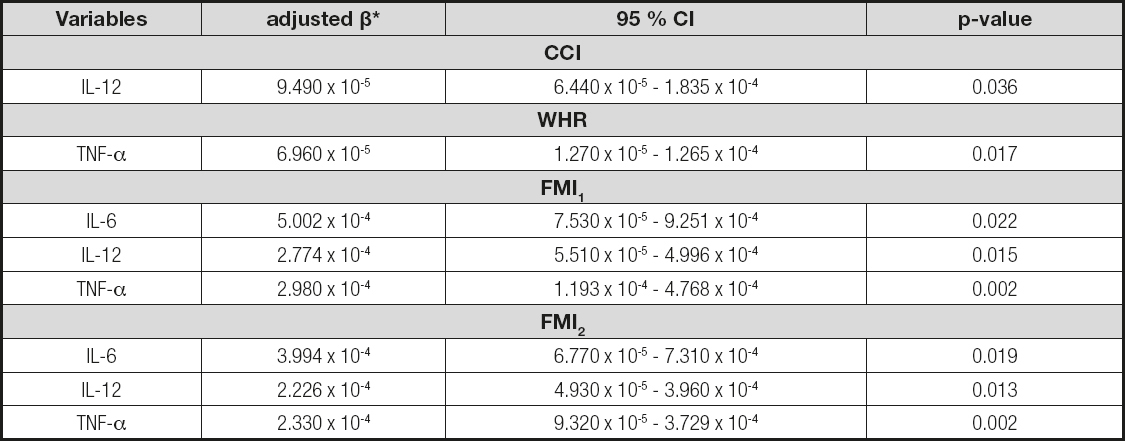
*Predictor variables adjusted according to family history of obesity. CCI: conicity index; WHR: waist-hip ratio; FMI1: fat mass distribution index 1; FMI2: fat mass distribution index 2; IL: interleukin; TNF-α: tumor necrosis factor alfa.
DISCUSSION
The higher prevalence of overweight and obesity is a fact in several countries worldwide, including Brazil. This body fat accumulation is a risk factor for diseases such as cardiovascular conditions, type- 2 diabetes and hypertension (23).The sample assessed showed that 46.70 % of subjects had a high percentage of body fat. When it comes to women, a body fat accumulation higher than expected is associated with risk for developing cardiometabolic diseases (5).
Another factor that deserves attention is body fat location, for the pattern of body fat distribution in women is related to estrogen levels, which tend to decrease over the years. It causes changes regarding where fat is stored, increasing the android region and, consequently, the risk of developing diseases (24). This implies that the risks for health are related to where body fat is stored. A higher level of android fat and/or its proportion in relation to gynoid fat, for instance, is linked to risk factors for developing diseases (25).
It is known that women tend to have a higher quantity of body fat than men. Most of the fat in a woman's body is located in the peripheral region (gynoid), while it is mostly found in the abdominal region in men (android) (26). This fact confirms the results found by the current study (Table III). Although gynoid fat is less associated with cardiometabolic risks when compared to android fat (27), 35.0 % of the sample had changes in waist circumference, which predispose to dyslipidemia (28).
Adipose tissue, mainly found in the central region of the body, has a significant role in the production of inflammatory cytokines. This capacity is even greater in intra-abdominal (visceral) adipose tissue when compared to subcutaneous adipose tissue (29). Visceral fat may produce higher levels of IL-6 and TNF-α in people with central obesity: that is, increased body fat, which may be identified by body fat indexes, can result in increased cytokine production (30).
Inflammatory parameters are indexes related to the risk of developing NCDs because they result from molecule secretion with inflammatory features, which is stimulated by the adipose tissue (31). This chronic inflammation tends to be triggered by excessive body fat, which increases circulating cytokines such as IL-6 and TNF-α (14).
The concentrations of IL-6 and TNF-α are directly proportional to the amount of adipose tissue (32). TNF-α is responsible for endothelial changes, phagocyte oxidative metabolism stimuli, and increased adipocyte activity. IL-6, produced by monocytes and endothelial cells, is responsible for increased levels of CRP, which is a marker of inflammation (15,16).
IL-6 can act in several ways in the body according to its concentration, and plays a role in the production of acute phase proteins, which are related to post-inflammatory response. Furthermore, its increase can be directly associated with body mass and insulin resistance, thus being related to subclinical inflammation in obesity (33).
TNF-α presents higher serum concentrations in individuals with high VAT in comparison to those without central obesity (34). This may be confirmed by the results found in this study (Table IV).
IL-12 plays a role in cell-mediated immunity, and is necessary to provide resistance to intracellular infection. Therefore, this is a cytokine that can be key in the development of autoimmunity, although conflicting data have been reported about it (35). The plasma concentrations of this cytokine are higher in individuals suffering from overweight or obesity in comparison to eutrophic ones. Hence, IL-12 correlates positively with fat mass.
A possible explanation for these high levels stems from the presence of hyperleptinemia in individuals with body fat changes (36). According to Fantuzzi (37), leptin favors the production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12 in monocytes and macrophages. Therefore, there is a relationship between weight accumulation and these inflammatory indexes. Furthermore, a correlation was identified between adiposity indexes and cytokines: increased IL-12, IL-6 and TNF-α levels are associated with increased CCI, WHR, FMI1 and FMI2 indexes (Table V).
CCI assesses the tendency towards developing cardiovascular and metabolic diseases, allowin direct comparisons of abdominal adiposity between individuals (38).
FMI1 and FMI2 play a role in defining metabolic syndrome and abnormalities in the distribution of body fat (lipodystrophy) (39). Changes in the distribution of body fat can be accompanied by changes in blood glucose, enhancing any predisposition to develop cardiovascular diseases and diabetes. Although these changes are prevalent in HIV-positive patients, they are unknown in healthy individuals. However, it is inferred that they arise from a sedentary lifestyle and consumption of hypercaloric foods (40). From this perspective, an assessment of FMI1 and FMI2 is useful for diagnosing and treating clinical obesity, as well as for identifying individuals at potential risk for developing risk factors related to obesity (39).
In the light of the foregoing, the models for multiple linear regression analysis between body adiposity indexes and cytokines in the current study (Table V) show that, as cytokines increase, so do these indexes. The calculation of these indexes is based on anthropometric measures, which are easier to obtain and have a lower cost as compared to cytokine assessment, since its application in clinical practice will lead to lower healthcare expenditures.
It is noteworthy that, when interpreting the data from this study, it is appropriate to consider certain research limitations. The key one is its cross-sectional design. Thus, the associations presented between independent factors and outcome variables do not necessarily represent causal relations, for this is the baseline of a longitudinal study. Therefore, the data shown can support further studies.
CONCLUSION
Pro-inflammatory cytokines in the current study were associated with an increase in adipose indexes. Obesity, especially morbid and visceral obesity, represents an inflammatory state, but its negative consequences are vitally important when it comes to cardiovascular disease. Therefore, these indexes may become a feasible strategy for clinical practice in order to identify propensity to inflammatory disorders.