INTRODUCTION
Hospital malnutrition is multifactorial (1), and its prevalence around the world is quite variable (2). In Latin America, malnutrition is present in 44.9 % of hospitalized patients (3). The prevalence reported in intensive care units (ICUs) in Latin America ranges from 65 % (3) to 74.1 % (4). Malnutrition favors the appearance of clinical complications (5) and implies higher financial costs for the healthcare system (6,7).
Patients in the ICU present a high risk of malnutrition because of the protein catabolism due to the inflammatory status (8,9). Additionally, studies have demonstrated an elevated prevalence of underfeeding (10,11).
The first step in the fight against hospital malnutrition is identifying patients with malnutrition or who are exposed to factors that could lead them to malnutrition. Nutritional screening identifies patients who would benefit from receiving adequate nutritional support to improve their prognosis. There are different nutritional screening tools (12); however, their use in ICU is difficult because of the unavailability and/or the invalidity of the data in those tools. The NUTRIC score is a screening tool especially designed for critically ill patients, including variables commonly available in the ICU that represent acute and chronic starvation, acute and chronic inflammation and the severity of illness (age, APACHE II, SOFA, number of comorbidities, days from hospital to ICU admission and interleukin-6 [IL-6]) (13).
International studies employing the modified NUTRIC score (without IL-6) have demonstrated that patients with high nutritional risk have worse clinical outcomes than low nutritional risk patients, and this could be modified by an improvement in energy and protein administration (14-16).
Nutritional support provides multiple benefits beyond supplying energy and protein requirements. It is able to modulate the metabolic response through the regulation of the immune response, preventing oxidative damage, maintaining the gut barrier integrity and favoring gut microbiota (17,18). Therefore, nutritional therapy should be considered as part of the treatment.
In our population, there are few studies focusing on the evaluation of nutritional risk and nutritional support in the ICU as well as its impact on the prognosis, which could be the basis for the implementation of interventional studies based on nutritional support. Therefore, our objective was to evaluate the association between nutritional risk and 28-day mortality and to characterize nutritional support in critically ill patients.
PATIENTS AND METHODS
STUDY DESIGN
A single-center, prospective cohort study was performed over 7 months in a medical-surgical ICU of a Mexican tertiary hospital. The study was approved by the Research Committee and the Research Ethics Committee of the hospital. The personal or family consent to participate in the study was obtained for every patient.
PATIENTS AND VARIABLES
The consecutive sample population consisted of adult patients (≥ 18 years) who were admitted to the ICU for ≥ 48 h, with available clinical and biochemical data and with personal or family consent to participate. Readmissions or patients with brain death diagnosis at admission were excluded.
Patients were clinically evaluated every day, and the study variables were complemented with biochemical, and nursing records. Nutritional risk was assessed with the modified NUTRIC score (mNUTRIC score) in the first 24 h of ICU admission (a score from 0 to 4 indicates low risk and a score from 5 to 9 indicates high risk).
The primary outcome was 28-day mortality. A phone call was made for those patients that were discharged from the hospital before the 28-day. Secondary outcomes were length of stay (LOS) in the ICU, LOS in the hospital and days on invasive mechanical ventilation (IMV), which were also recorded from the first day in the ICU until 28-day. Only patients with IMV for ≥ 24 h were included as ventilated patients.
Characteristics of nutritional support (route of administration, nutritional adequacy and starting day) were collected during the ICU stay and for a maximum of 12 days. To obtain the daily and 12-day nutritional adequacy, calories (including propofol calories) and protein administration were recorded every day to determine the percent of calorie and protein requirements received. Energy requirements were calculated with the weight-based equation 25-30 kcal/kg using the patients’ actual body weights when their body mass index (BMI) were < 25 and their ideal body weights when their BMIs were > 25. It was considered that calorie requirements were achieved when patients received at least 80 % of the corresponding requirements. If they received > 110 %, it was considered overfeeding, and if it was less than 80 %, it was underfeeding. The protein requirements were 1.2-2 g/kg when the BMI was < 30, 2 g/kg ideal body weight when the BMI was 30-40 and 2.5 g/kg ideal body weight when the BMI was > 40.
STATISTICAL ANALYSIS
Statistical analysis was performed with IBM SPSS STATISTICS 23 software. Data are reported as the means and standard deviation (SD), medians and interquartile ranges (IQRs) or percentages and frequencies. Differences between high and low nutritional risk and between surviving and non-surviving patients were assessed with the Chi-squared or Fisher exact test and with Student’s t-test or the Mann-Whitney U test. The relative risk (RR) with a 95 % confidence interval (95 % CI) was calculated for 28-day mortality in high nutritional risk patients, as well as the Log-rank (Mantel-Cox) Test to compare survival curves between groups.
The correlations between the mNUTRIC score and LOS in the ICU, LOS in the hospital and days with IMV were evaluated with the Spearman correlation coefficient.
The predictive capacity of the mNUTRIC score for 28-day mortality was assessed by the area under the receiver operating characteristic curve (AUC-ROC).
Furthermore, mNUTRIC score sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated for 28-day mortality.
A p-value < 0.05 was considered statistically significant.
SAMPLE CALCULATION
Considering the published results (19), a total of 110 patients were necessary to find a difference of 21.4 % in 28-day mortality with an α of 0.05 and a β of 0.20.
RESULTS
In total, 352 patients were admitted to the ICU during the study period and 110 patients met the inclusion criteria. Patients with a LOS in the ICU < 48 h (n = 83), without complete data (n = 121), without consent (n = 2), with a brain death diagnosis at admission (n = 15) and readmissions (n = 21) were excluded.
The diagnoses were cardiac surgery 28.2 % (n = 31), sepsis or septic shock 20.9 % (n = 23), neurological surgery 12.7 % (n = 14), neurological procedures 7.3 % (n = 8), neurologic disease 5.5 % (n = 6), hypovolemic shock 3.6 % (n = 4), Guillain-Barrésyndrome 2.7 % (n = 3), cardiogenic shock 2.7 % (n = 3), and others 16.4 % (n = 18). Patients were 50.7 ± 16.8 years of age, had a mean APACHE II score of 15.5 ± 5.8, and had a mean SOFA score of 6.9 ± 3; IMV was required in 65.5 % (n = 72), and 28-day mortality occurred in 23.6 % (n = 26) of the patients. High nutritional risk according to the mNUTRIC score was found in 35 patients (31.8 %).
Comparisons between low- and high-risk patients are shown in table I. There were significant differences in age (47.1 ± 15.8 vs. 58.4 ± 16.7, p = 0.001), APACHE II score (12.8 ± 4.3 vs. 21.3 ± 7.1, p < 0.001), SOFA score (5.7 ± 2.4 vs. 9.6 ± 2.4, p < 0.001), mNUTRIC score (3 [2-4] vs. 6 [5-7], p < 0.001), proportion of patients with IMV (57.3 % vs. 82.9 %, p = 0.009) and proportion of deaths (9.3 % vs. 54.3 %, p < 0.001). Patients with high nutritional risk had a higher risk of 28-day mortality (RR 5.81, 95 % CI 2.69-12.53) in comparison with those with low nutritional risk (Fig. 1). No differences in LOS and days with IMV were found between the study groups.
Table I. Patient characteristics

Mean ± SD or median and interquartile rank.APACHE II: Acute Physiology and Chronic Health disease Classification System II; SOFA: Sequential Organ Failure Assessment; BMI: Body mass index; LOS: length of stay; IMV: invasive mechanical ventilation.
aChi-squared; bStudent’s t-test; cMann-Whitney U test.

Figure 1. Kaplan-Meier survival curve between patients with low and high nutritional risk according to the mNUTRIC.
The mNUTRIC score (0-9) AUC for the prediction of 28-day mortality was 0.795 (95 % CI 0.692-0.898), while for the APACHE II score it was 0.777 (95 % CI, 0.673-0.881) and for the SOFA score it was 0.706 (95 % CI, 0.598-0.813) (Fig. 2).

Figure 2. Receiver operating characteristic curve for 28-day mortality. A. ROC curve of the mNUTRIC score for 28-day mortality. B. Sensitivity, specificity and predictive values for different mNUTRIC score to predict 28-day mortality. C. ROC curve of the APACHE II score for 28-day mortality. D. ROC curve of the SOFA score for 28 days.
A mNUTRIC score ≥ 5 had a sensitivity of 73.1 % (95 % CI 53.9-86.3 %), specificity of 81 % (95 % CI, 71.3 %-87.9 %), positive predictive value of 54.3 % (95 % CI 38.2 %-69.5 %) and negative predictive value of 90.7 % (95 % CI, 82 %-95.4 %) for 28-day mortality. Higher mNUTRIC score had a better specificity while lower score had better sensitivity (Fig. 2B).
When we compared the characteristics between survivors and non-survivors at 28-day (Table I), we found significant differences in age (48.8 ± 16.5 vs. 56.6 ± 16.8, p = 0.039), APACHE II score (14.2 ± 5.4 vs. 19.6 ± 5.3, p < 0.001), SOFA score (6.4 ± 3 vs. 8.5 ± 2.5, p < 0.001), mNUTRIC score (3 [2-4] vs. 5 [4-6.3], p < 0.001) and proportion of patients with IMV (55.9 % vs. 96.2 %, p < 0.001). In the group of survivors, there were positive correlations between the mNUTRIC score (0-9) and the LOS in the ICU (r = 0.216, p = 0.049), LOS in the hospital (r = 0.230, p = 0.036) and days with IMV (r = 0.306, p = 0.037).
Regarding the feeding, oral intake was possible in 39.1 % of the patients (n = 43), 5.5 % (n = 6) fasted during their entire ICU stay, and nutritional support was implemented in 55.4 % (n = 61) of the patients. Nutritional support was initiated in the first 48 h after ICU admission in 52 patients (85.2 %).
The characteristics of enteral nutrition (EN), parenteral nutrition (PN) and enteral + parenteral nutrition (EN + PN) are shown in table II. Patients received, on average, 52.9 % and 46.0 % of their energy and protein requirements, respectively. Underfeeding occurred in 73.8 % (n = 45) and overfeeding in 8.2 % (n = 5) of the patients. Only 11 patients (18 %) received between 80 and 110 % of their nutritional requirements. Regarding protein intake, only 21.3 % (n = 13) of the patients met the nutritional requirements (Fig. 3).
Table II. Characteristics of nutritional support
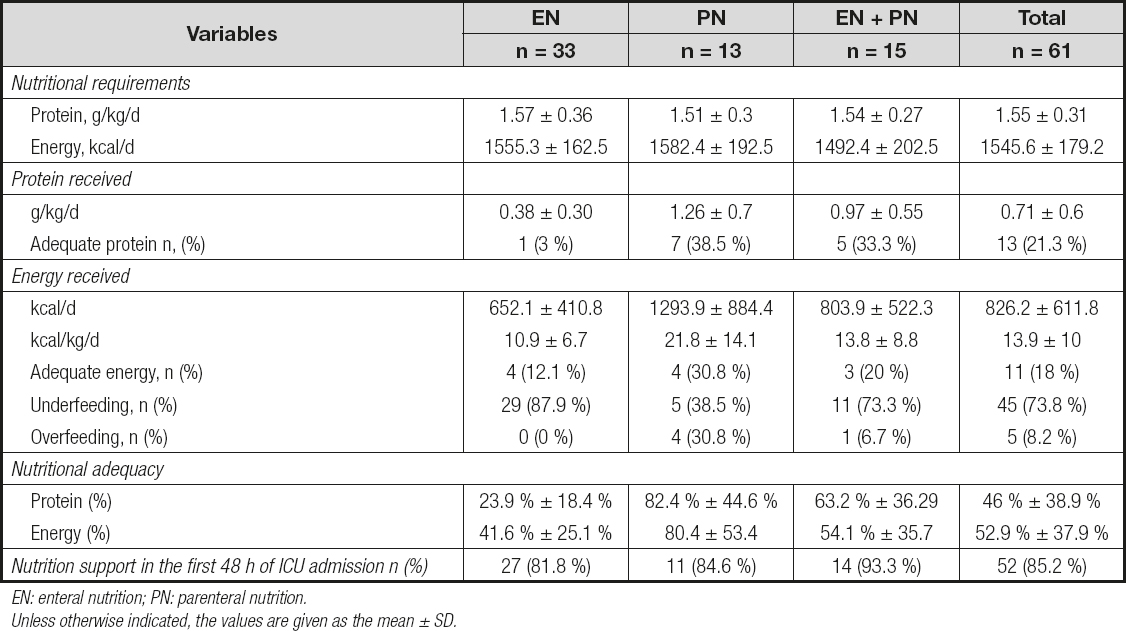
EN: enteral nutrition; PN: parenteral nutrition.Unless otherwise indicated, the values are given as the mean ± SD.

Figure 3. Daily nutritional adequacy in patients with nutritional support. A. Nutritional adequacy in all patients with nutrition support. B. Nutritional adequacy in patients with enteral nutrition. C. Nutritional adequacy in patients with parenteral nutrition. D. Nutritional adequacy in patients with enteral and parenteral nutrition.
No differences were found in the energy and protein administration between survivors and non-survivors (597 kcal/day [317-1011] vs. 693kcal/day [415-1585] p = 0.273 and 0.55 [0.25-0.85] g/kg/day of protein vs. 0.61 [0.22-1.33] g/kg/day p = 0.462).
DISCUSSION
In this Mexican ICU in a specialty hospital, we followed a cohort for 28 days and found that nutritional risk, assessed with the mNUTRIC score, was associated with 28-day mortality. In surviving patients, there were positive correlations between the mNUTRIC score and the LOS in the ICU, LOS in the hospital and days with IMV. Furthermore, we found a high prevalence of underfeeding among patients receiving nutritional support.
The prevalence of high nutritional risk found in our study (31.8 %) is lower than in other studies. An international multicenter study reported a global prevalence of 57 % (10). That study found that in Latin America, the prevalence was 53 %. Later, other authors reported prevalence greater than 40 %. (15,19 20 21 22 23 24-25) One of the reasons for this difference could be the characteristics of our population, which is on average younger and with less severe disease than the populations in other studies. This is important because the mNUTRIC score includes age and severity of illness as part of its variables.
Although our patients had less severe disease, the 28-day mortality that we found (23.6 %) was similar to what has been reported in other ICUs (19,26). This result probably reflects the fact that mortality outside the ICU is multifactorial (27) and that patients can be discharged with sequelae (post-intensive care syndrome) (28). The recovery from the critically ill patients involves physical, cognitive and psychiatric elements, and some of them do not succeed.
In our study, the patients with high nutritional risk had an almost 6 times higher 28-day mortality risk than those with low nutritional risk. The comparison between the results of this sample population and other published data are shown in table III (15,19,26). Some of these data had already been calculated by the authors of the original studies, and others were calculated by the authors of this study with the available information. The RR from these studies was lower than in our study, which is due to the high mortality we found in the high nutritional risk group (more than half of our high nutritional risk patients died).
Table III. Data comparison between other published studies and this sample. Relative risk and diagnostic ability of mNUTRIC score > 5 for 28-day mortality

RR: relative risk; PPV: positive predictive value; NPV: negative predictive value; LR+: positive likelihood ratio, LR-: negative likelihood ratio.Some of these data had already been calculated by the authors of the original studies, and others were calculated by the authors of this study with the available information.
According to the NUTRIC score validations, the mortality of high nutritional risk patients decreases if they receive appropriate nutrition support in the ICU (14,15). Beyond that, there are some nutritional factors we could control before and after the ICU admission. For example, a large portion of our sample (40.9 %) was composed of patients with major surgical interventions and almost all were elective procedures, which means that these patients have time to be prepared with nutritional protocols before the surgery; however, in our population, that is not common practice. Moreover, nutritional evaluations should be performed when patients are discharged from the ICU and from the hospital to ensure the meeting of nutritional requirements, which is not easy because the hypermetabolism continues for months and many times the patients face gastrointestinal problems. In the end, the recovery of their muscle mass, force and quality of life depends in part on adequate nutrition.
We did not find significant differences in the LOS in the ICU, the LOS in the hospital and days with IMV between high- and low-risk patients. Other works have reported differences, with high-risk patients having more days in the ICU and in the hospital and more days with IMV (15,19). This likely occurred due to our small sample size. However, we found positive correlations between the mNUTRIC score and the LOS in the ICU, LOS in the hospital and days with IMV only in the surviving patients.
The mNUTRIC score was useful for predicting 28-day mortality; it was even slightly better than the APACHE II and SOFA scores. Our result was similar to what has been reported by other studies (15,19,26). However, some works have found a very low predictive ability of the mNUTRIC score (14,29).
The mNUTRIC score ≥ 5 was the best cut-off point for us to predict the 28-day mortality, and the specificity and positive predictive value were higher than the values in other studies (15,19,26) (Table III).
We found a high prevalence of underfeeding, which is common in ICU (10,11). The energy and protein adequacy that we found is similar to what has been reported by others (30-33). Despite the controversy around the amount of energy that these patients should receive and based on current available evidence, the international guidelines (34,35) advise avoiding underfeeding and overfeeding because both have been associated with worse outcomes.
Protein administration is more important than energy administration because of the large loss of body protein and the need to restore tissues and maintain immune function. We found that only 21.3 % of the patients with nutrition support reached the minimum protein requirements. On average, our patients received 0.71 g/kg of protein, which is below the recommendations (34-36). We did not find differences in energy or protein intake between survivors and non-survivors, although there was a trend to a lower energy and protein administration in survivors, this data should be cautiously considered since the sample size in the non-survivors group is much lower than in the survivors groups, which could partially explain a higher variability in the measurements; besides that, the study was not powered-designed to clarify this difference. Together with this, it would be also possible that Nutritionist and Physicians in charge of these patients tended to prescribe higher nutritional support to them due to the higher disease severity, in an effort to improve patients´ health. However, standardized and comparative clinical protocols are needed to deeply evaluate the impact of a higher nutritional support on these patients. Also, it is important to take into account that despite the evidence that indicate benefits from approaching nutritional goals (30,37,38), there is still controversy around the nutrition support that critically ill patients should receive. We are aware that some studies found that higher nutritional adequacy is associated with worst outcomes (39). But this topic is more complex than just prescribing calories and protein. It is necessary to consider that there are unanswered questions about other nutrition variables that could impact patient´s prognosis, such as the optimal timing to initiate and the optimal rate of increase calories and protein (40). Nutritional support should be considered as a tailored suit for each patient.
Although some studies have found that patients in the ICU tend to start enteral nutrition after the first 48 h of admission (31,37,41), in our case, more than 80 % of the patients had early enteral nutrition, as reported by Yeh et al. (42). This route of administration was the most common, as in other studies (10,30,33), and it is in line with the recommendation of preferring the enteral route when possible because it can benefit the gut barrier integrity, the microbiota and immunity.
We observed that patients with PN met their nutritional requirements more easily than patients with only EN or EN + PN, but at the same time, there were more overfed patients in this group. In addition, all the overfed patients died, but we cannot affirm that these patients died because of this. On the other hand, most underfed patients received EN, which denotes that the exclusive use of this feeding route sometimes may not help or may not be enough to achieve nutritional goals in critically ill patients, and alternative ways should be implemented depending on the particularity of each patient. The EN group had the worst protein and energy levels. Multidisciplinary efforts should be made to avoid underfeeding and overfeeding.
Among the strengths of this study is that it was a prospective and longitudinal design with a sample size calculation and that we included medical and surgical patients. To our knowledge, this is one of the few studies to report the results of screening with the mNUTRIC score and the characteristics of nutrition support in the Mexican ICU population. During the recording of nutritional support characteristics, we considered the calories coming from propofol, which usually represent a large part of the total calories administered.
The limitations include the fact that we only associated nutritional risk with 28-day mortality, LOS in the ICU, LOS in the hospital and days with IMV. It would have been interesting to evaluate the associations with quality of life and functionality variables after the ICU stay because the goal is not only that the patients are discharged alive.
This was a single-center study and our sample size was too small to find associations with the secondary variables.
Regarding the screening tool we used, it is important to consider that there are published studies that do not validate NUTRIC score as a nutrition screening tool. In the post-hoc analysis of PermiT trial (22) no difference in mortality was found among patients at high and low risk who received permissive underfeeding and standard feeding. This means that NUTRIC score could be more useful as a disease severity score than as a nutritional risk screening tool. Indirect calorimetry, which is the gold standard for energy prescription, was not used, so we were not able to evaluate the changes in the energy requirements. Moreover, we did not record the calories from intravenous glucose solutions; although they do not usually contribute significantly to the total calories, our estimation of caloric administration is not 100 % accurate.
Further research is necessary to identify the factors involved in the failure to achieve the nutritional goals, as well as to better understand the effects of nutritional support on the outcomes. In our study, we cannot say that high-risk patients would benefit from adequate nutritional support, as in the validations of the NUTRIC score. It is very difficult to extrapolate the results from the available studies to all ICU patients. In addition, the participation of Latin America in studies related to nutritional support in critical illness is very low; therefore, we only have the option to adopt the results from other countries. It is time to focus on this area of nutrition in our environment.
CONCLUSION
High-risk patients assessed with the mNUTRIC score had a higher risk of 28-day mortality. Among survivors, the higher the mNUTRIC score was, the longer the ICU and hospital stays and the IMV duration. Further studies are needed in our population to verify that a better nutritional adequacy in high nutritional risk patients is able to impact prognosis, as in other scenarios. The majority of patients receiving nutritional support had it initiated early, but less than a quarter of the patients reached the energy and protein requirements. Strategies must be applied to follow the guidelines regarding nutritional support.














