INTRODUCTION
Sarcopenia is a progressive and generalized skeletal muscle disorder that is associated with increased adverse events, including falls, fractures, physical disorders, and mortality (1). In individuals with chronic kidney disease (CKD), sarcopenia is prevalent and associated with increased morbidity and mortality and the occurrence of cardiovascular complications (2).
Several factors have been described as determinants of sarcopenia, such as poor nutritional status, physical inactivity, metabolic and endocrine function disorders, chronic diseases, insulin resistance, and inflammation (3,4). It has been suggested that skeletal muscle is a secretory organ that produces and releases proteins with specific endocrine functions in an attempt to mediate a protective effect on catabolism caused by inflammation. These proteins seem to stimulate muscle growth and hypertrophy, increase lipid oxidation, and induce anti-inflammatory activity (5,6).
Inflammation has proved to be the most important predictor of sarcopenia (7). Among the causes of inflammation in hemodialysis patients are the dialysis procedure itself and factors not related to dialysis, such as the presence of comorbidities, clinical complications, genetic factors, and diet (9). Studies suggest that increased inflammatory status, characterized by increased serum C-reactive protein (CRP) and proinflammatory cytokines, inhibits appetite and causes increased protein catabolism, lipolysis, and energy expenditure, negatively impacting the nutritional status of these patients (10,11).
Persistent inflammation is a feature of advanced CKD that promotes a prematurely aged phenotype and plays a central role in sarcopenia, predicting poor clinical outcomes (12,13). These findings suggest that there is a link between inflammatory mediators, muscle mass, and muscle function, but few studies have explained this link (14).
Muscle protein imbalance, increased activation of the ubiquitin-proteasome pathway, alteration of mitochondrial dynamics, cell death, increased muscle adiposity, and synthesis that affects insulin resistance and restricted muscle repair and regeneration have been suggested as mechanisms underlying muscle degeneration induced by inflammation (15).
Thus, it has been difficult to determine and understand the magnitude of this problem and the consequences of uremic inflammation in hemodialysis (HD) patients because the mechanisms of how inflammation interacts with biomarkers varies, and the relationship between biomarkers and outcomes are misinterpreted as changes in body composition, such as sarcopenic obesity (13,18). In this context, it is relevant to determine the relationship between sarcopenia, inflammation, and body composition in chronic renal patients undergoing HD.
MATERIALS AND METHODS
The present cross-sectional study enrolled chronic renal patients treated in the five existing hemodialysis centers in the city of São Luís, Maranhão. Individuals were recruited from centers according to the following criteria: were older than 20 years of age who received regular hemodialysis for at least three months; patients underwent treatment 3 times a week, with each session lasting between 3.5 and 4 hours, and had serum ultra-sensitive C-reactive protein (US-CRP) assessed. Non-inclusion criteria were as follows: pregnant women, amputees, carriers of neurological diseases and/or sequelae of stroke, patients with cognitive or intellectual impairment that prevented them from responding to the form, and those with cancer and acquired immunodeficiency syndrome.
The study was approved by the Committee on Ethics in Research of the Federal University of Maranhão University Hospital with the assent number 1.232.730/2015; it fulfilled the ethical recommendations for research involving human beings and was in accordance with the World Medical Association and the Helsinki Declaration.
In 2015, according to a survey conducted in dialysis centers, 1,009 patients underwent HD in the city of São Luís. The sample size was calculated considering 694 eligible patients; correlation between the level of hsCRP and sarcopenia of at least 0.20, significance level of 0.05, and power of 0.80 were considered to detect a linear correlation. The minimum required sample size was 195 patients. Ten percent were added to compensate for possible losses, yielding a final sample size of 209 patients. In each center, sample size was obtained through stratified random sampling proportional to the population size, considered here as the number of HD patients in each center.
Data were collected from February to December 2016. Demographic, socioeconomic, and clinical data were collected through the administration of forms and laboratory tests, which were collected from the medical records.
Laboratory tests were conducted just prior to patient evaluations and included the following: creatinine (via Jaffé reaction; Cobas® 6000 analyzer series, Roche Diagnostics, Indianapolis, IN, USA), albumin (bromocresol green), hemoglobin (calorimetry by spectrophotometer; ADVIA® 120 System, Siemens Healthineers, Erlangen, Germany), 25-hydroxyvitamin D (Cobas® 6000 analyzer series, Roche Diagnostics), and serum hsCRP levels (Cobas® 6000 analyzer series, Roche Diagnostics). Inflammation was diagnosed in patients with hsCRP serum levels ≥ 0.3 mg/dL. Blood samples were collected following the department’s routine during the second HD session of the first week of the month.
The demographic and socioeconomic characteristics of interest were as follows: age (years), duration of HD (< 1 year, 1 to 5 years, > 5 years), sex, place of residence (São Luís or interior of the State), self-reported skin color (white, brown/black/mulatto, oriental, indigenous, unknown), education (years of education), income (minimum wage: less than or equal to 1 salary, 2 to 3 wages, and ≥ 4 wages), as well as clinical data such as presence of comorbidities and underlying disease.
An anthropometric and functional evaluation was performed 15 minutes after HD and always on the second dialysis session of the week to avoid the influence of fluid retention on measurement. Weight (scale, Filizola®, SP, Brazil) and height (stadiometer, Alturexata®, Alturexata Inc., Belo Horizonte, Minas Gerais, Brazil) were measured for the subsequent calculation of the body mass index (BMI).
To assess body composition, appendicular skeletal muscle mass (ASM), lean mass (kg), and body fat percentage (% fat), double-energy X-ray absorptiometry (DEXA) via the Lunar Prodigy Primo system (GE Healthcare, Chicago, IL, USA) was used with daily calibration as requested by the manufacturer. The evaluation was performed within 24 hours following the HD session.
The ASM was corrected by height, generating the appendicular skeletal muscle mass index (ASMI) using the formula: appendicular lean mass (ALM) / height². Lean mass decreased when ASMI values were < 7.0 kg/m² for men and 5.5 kg/m² for women.
Skeletal muscle functionality was evaluated through hand grip strength (HGS) (Jamar® Smedley dynamometer, Stoelting Company, Wood Dale, IL, USA), and physical performance was measured using the 4-meter gait speed test. HGS was measured on the arm opposite the fistula. Three readings were performed with a one-minute interval between them, and the highest value was recorded. An HGS < 27 kg for men and < 16 kg for women was considered as reduced strength. The usual gait speed was measured by walking time on a 4-meter course, with values ≤ 0.8 m/s being considered an indicator of low performance.
The criterion adopted by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) for low muscle function and muscle mass was used for identifying patients with sarcopenia (1).
A descriptive analysis of categorical variables was performed using frequency and percentage, and quantitative variables were analyzed using the mean and standard deviation (mean ± SD) or median and interquartile range. The distribution of normality was checked with the Shapiro-Wilk test. Quantitative variables were compared using Student’s t-test or Mann-Whitney tests according to the distribution of normality. The chi-squared test or Fisher’s exact test was used for comparison of proportions. The statistical significance level was set at 5 %.
A Poisson regression with a robust error variance was fit to assess the association between sarcopenia and inflammation. The dependent variable was the presence of sarcopenia. The independent variables analyzed in the model as possible confounding variables were: age, gender, diabetes (DM, yes or no), HD time, BMI, % fat, albumin, hsCRP, and vitamin D.
A stepwise forward selection was used to progressively discard the variables not associated with sarcopenia. Variables with a p-value ≤ 0.20 were retained as part of the model. The final Poisson regression model included all variables with p < 0.05.
The prevalence ratio (PR) and 95 % confidence intervals (95 % CI) were estimated to measure the magnitude of the association between sarcopenia and inflammation (measured by the numerical variable hsCRP). All analyses were performed using the Stata 14 software (StataCorp., College Station, TX, USA).
RESULTS
The study sample consisted of 209 chronic renal patients undergoing HD. Most patients were men (59.3 %) with a mean age of 51.9 ± 15.0 years, living in São Luís, MA (68.4 %), of black or brown skin color (88.0 %), with less than nine years of education (48.3 %), and income between two and four minimum wages (59.2 %) (data not shown in table).
Most patients reported between 1 and 4 years of hemodialysis treatment (59.8 %). Arterial hypertension (37.5 %) was the most frequent underlying disease, followed by DM (19.0 %). About 50.2 % of patients had inflammation (hsCRP > 0.3 mg/dL). Muscle mass was low in 41.3 % of patients, and muscle strength was low in 51.9 % of these patients. The prevalence of sarcopenia was 29.2 %, considering the EWGSOP2 criteria (1). The prevalence of sarcopenia among men was 41.9 %, and 10.6 % among women (data not shown in table).
Compared to men without sarcopenia, it was observed that the sarcopenia group was older (aged 59.7 ± 17.1 years vs. 50.1 ± 12.7 years, respectively; p = 0.005), had lower BMI (22.0 ± 3.6 kg/m2 vs. 24.3 ± 3.3 kg/m2, respectively; p < 0.001), HGF (19.4 ± 4.5 kg vs. 30.3 ± 8.8 kg, respectively; p < 0.001), and lean mass (40.6 ± 5.6 kg vs. 48.8 ± 6.1 kg, respectively; p < 0.001), and worse physical performance status as measured by gait speed (0.8 ± 0.2 m/s vs. 1.0 ± 0.2 m/s, respectively; p < 0.001). Among women, compared to the group without sarcopenia, the group with sarcopenia had lower BMI (25.1 ± 4.5 kg/m2 vs. 20.4 ± 2.8 kg/m2, respectively; p = 0.002), HGF (17.2 ± 5.8 kg vs. 11.3 ± 3.3 kg, respectively; p < 0.001), lean mass (37.6 ± 5.8 kg vs. 30.1 ± 2.6 kg, respectively; p = 0.003), and worse physical performance (1.0 ± 0.2 m/s vs. 0.6 ± 0.1 m/s, respectively; p = 0.002) (Table I).
Table I. Demographic characteristics, body composition and functionality in chronic renal patients undergoing hemodialysis, according to sarcopenia and sex (n = 209)
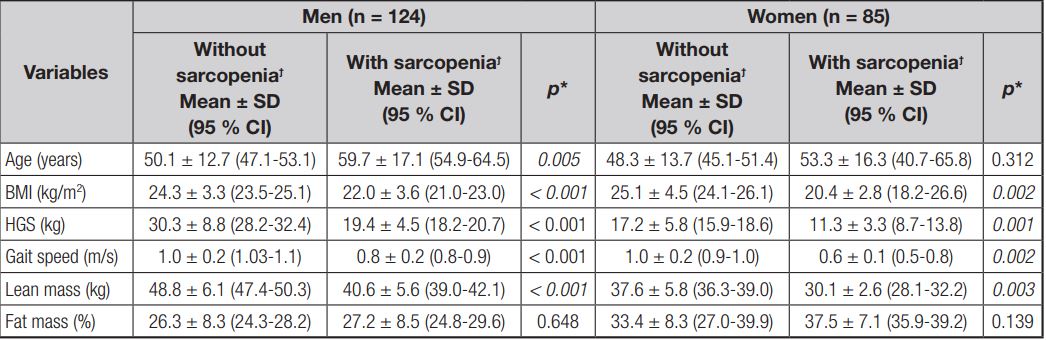
BMI: body mass index; HGS: hand grip strength. *Paired Student’s t-test or Wilcoxon’s test, as appropriate. ϯSarcopenia diagnosed by revised European consensus on definition and diagnosis.
With regard to clinical and laboratory characteristics, men with sarcopenia vs. those without sarcopenia had lower levels of serum creatinine (9.9 ± 3.0 mg/dL vs. 12.3 ± 4.0 mg/dL, respectively; p = 0.006), and hemoglobin (10.5 ± 1.6 mg/dL vs. 11.4 ± 2.3 mg/dL, respectively; p = 0.002) and higher levels of hsCRP (1.5 ± 2.7 mg/dL vs. 0.4 ± 0.5 mg/d, respectively; p = 0.005). Women did not show statistically significant differences in any of the evaluated parameters (Table II).
Table II. Laboratory and clinic characteristics in chronic renal patients undergoing hemodialysis according to sarcopenia and sex (n = 209)

hsCRP: high-sensitivity C-reactive protein; 25(OH)D: 25-hidroxi-vitamin D; HB: hemoglobin; DM: diabetes mellitus; HD: hemodialysis. *Paired Student’s t-test or Wilcoxon’s test, as appropriate. ϯSarcopenia diagnosed by revised European consensus on definition and diagnosis.
Inflammation was observed in older men (57.7 ± 15.3 vs. 50.9 ± 14.9; p = 0.013) and individuals with lower HGF (23.8 ± 8.2 kg vs. 27.2 ± 9.5 kg; p = 0.031) and ASMI (6.7 ± 1.0 kg/m2 vs. 7.1 ± 0.9 kg/m2; p = 0.044) (Table III). Furthermore, a higher prevalence of sarcopenia was observed among men with inflammation compared to non-inflamed men (52.5 % vs. 32.3 %, respectively; p = 0.023) (Table IV). Women with inflammation compared to those without inflammation had a higher BMI (26.0 ± 4.8 kg/m2 vs. 22.9 ± 3.7 kg/m2, respectively; p = 0.006) and % body fat (38.5 ± 8.0 % vs. 35.4 ± 6.0 %, respectively; p = 0.014), and lower lean mass values (34.9 ± 6.0 kg vs. 38.5 ± 5.6 kg, respectively; p = 0.006), ASMI (5.8 ± 0.1 kg/m2 vs. 6.4 ± 1.0 kg/m2, respectively; p = 0.003) (Table III), and serum levels of vitamin D (31.3 ± 6.8 vs. 39.3 ± 10.4 μg/dL, respectively; p = 0.007) (Table IV).
Table III. Demographic characteristics, body composition, and functionality in chronic renal patients undergoing hemodialysis according to inflammation and sex (n = 209)

BMI: body mass index; HGS: hand grip strength; ASMI: relative appendicular skeletal muscle mass index. *Paired Student’s t-test or Wilcoxon’s test, as appropriate. ϯInflammation diagnosed by high-sensitivity C-reactive protein > 0.3 mg/dL.
Table IV. Laboratory and clinic characteristics in chronic renal patients undergoing hemodialysis according to inflammation and sex (n = 209)

25(OH)D: 25-hidroxi-vitamin D; HB: hemoglobin DM: diabetes mellitus; HD: hemodialysis. *Paired Student’s t-test or Wilcoxon’s test, as appropriate. #Sarcopenia diagnosed by the revised European consensus on definition and diagnosis. ϯInflammation diagnosed by high-sensitivity C-reactive protein > 0.3 mg/dL.
The Poisson regression model showed that sarcopenia is associated with increased hsCRP values (PR = 1.06; p = 0.005). In the unadjusted analysis, sarcopenia was associated with males (PR = 7.52; p < 0.001), age (PR = 1.02; p = 0.007), BMI (PR = 0.76; p = 0.007), presence of DM (PR = 1.61; p = 0.079), vitamin D (PR = 0.99; p = 0.481), and % body fat (PR = 1.07; p < 0.001) (Table V).
Table V. Unadjusted analysis of sarcopenia in chronic renal patients undergoing hemodialysis (n = 209)
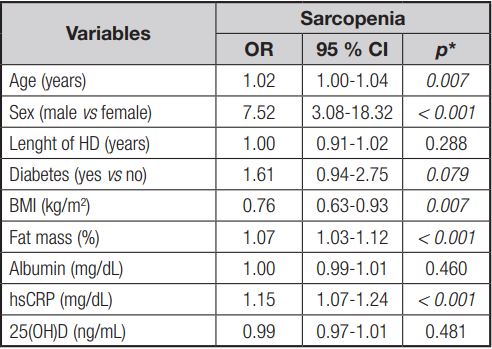
HD: hemodialysis; BMI: body mass index; hsCRP: high-sensitivity C-reactive protein; 25(OH)D: 25-hidroxi-vitamin D; OR: oddis ratio. *Poisson regression.
Table VI. Adjusted analysis of sarcopenia in chronic renal patients undergoing hemodialysis (n = 209)

BMI: body mass index; hsCRP: high-sensitivity C-reactive protein; OR: oddis ratio. *Poisson regression.
After the adjusted analysis, the following factors continued to be associated with sarcopenia: males (PR = 5.7; p < 0.001), presence of DM (PR = 1.87; p < 0.001), increased age (PR = 1.02; p < 0.001), BMI (PR = 0.74; p < 0.001), hsCRP (PR = 1.06; p = 0.005), and % body fat (PR = 1.07; p < 0.001).
DISCUSSION
In this study, the prevalence of sarcopenia was 29.2 %, considering the EWGSOP2 criteria. The prevalence of inflammation was 50.2 % considering hsCRP ≥ 0.3 mg/dL, confirming the high prevalence of these conditions in chronic renal patients undergoing hemodialysis. Sarcopenia was associated with inflammation, presence of DM, male gender, older age, and higher BMI and % body fat values.
Inflammation in HD patients is caused by multiple factors, including those related to uremia, comorbidities, and the dialysis procedure itself (17). CKD is characterized by high circulating levels of inflammatory markers such as CRP, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Additionally, inflammation is an important cause of muscle mass loss in this population (19) as it causes severe tissue proteolysis. Higher serum CRP concentrations are associated with physical disability, mortality, and reduction in muscle strength, and are correlated with lower muscle mass in older individuals (20,21).
In a meta-analysis involving 16 studies with 3,072 people with sarcopenia and 8,177 controls, individuals diagnosed with sarcopenia were significantly more likely to have higher levels of CRP (20). This process may be more severe among vulnerable populations, such as those with CKD at the final stage of the disease, because this condition is associated with decreased protein synthesis and increased protein degradation, leading to sarcopenia regardless of age (22).
Deger et al. evaluated 129 adults receiving HD in the United States and found a similar result to the present study; that is, there is an association between inflammation and sarcopenia, concluding that inflammation is a strong and independent determinant of protein homeostasis in skeletal muscle in HD patients (23).
This study found that 50.2 % of patients had inflammation according to hsCRP levels. Stenvinkel et al. (2016) analyzed a cohort of 5,904 HD patients from 15 European countries and confirmed a high prevalence of inflammation in HD patients (54 %) classified as increased CRP and/or decreased albumin. They also found that inflamed men were older, showed lower strength, lower skeletal muscle mass, and a higher percentage of sarcopenia compared to non-inflamed men (24).
Age-induced inflammation is a low-grade and continuous type of inflammation with increased CRP levels and reduced levels of anti-inflammatory cytokines, described as immunosenescence. It occurs in older adult individuals and is associated with diseases, sarcopenia, and even mortality (25).
Inflamed women had higher BMI and % fat and a lower amount of vitamin D, lean mass, and skeletal muscle mass when compared to non-inflamed women. The fact that adipose tissue has several functions besides storing energy may explain the relationship between percentage of fat and inflammation among women, as adipose tissue is responsible for the synthesis of IL-6, which stimulates CRP production. Thus, it is expected that its concentration will be higher in individuals with excessive body fat, which was confirmed by the findings of this study.
Moreover, the presence of obesity and lower lean mass, which are characteristic of sarcopenic obesity, may be a feedback system for these two pathological conditions. Obesity involves a pro-inflammatory state, detected by the presence of cytokines that directly interfere with muscle integrity; in addition, excess adipose tissue and fat infiltration in skeletal muscle mass interferes with muscle function (26).
The inflammatory status of renal patients is also associated with anemia caused by resistance to the medullary action of erythropoietin (EPO), which corroborates the findings of the present study. A mechanism underlying this association may be the action of inflammatory cytokines on erythropoietin progenitor cells, opposing EPO and then stimulating apoptosis (27).
The prevalence of sarcopenia in this study was 29.2 %, considering the EWGSOP2 criteria. Isoyama et al. (2014) used DEXA and the diagnostic criteria recommended by the EWGSOP in 2010 in a cohort of 330 Swedish dialysis patients with a mean age of 53 ± 13 years, and found a lower prevalence of sarcopenia (20 %) (28). Depending on the criteria used, Rosa (2017) found a variation of 13 % and 36 % in the prevalence of sarcopenia in 67 patients with a mean age of 54.6 ± 14.6 years, and showed that all the criteria studied showed moderate-to-high agreement among them (29).
Authors report that the cutoff points for defining muscle mass, the diagnostic methods used, the population studied (dialysis or pre-dialysis), and age may explain the differences found between the prevalence values of this syndrome among several studies (28,30-32). Seeking standardization, the EWGSOP published an update of the 2010 consensus in 2019 considering the scientific and clinical evidence over the previous decade, updating the definition and cutoff points of sarcopenia for the general population. However, this consensus did not define cutoff points specific to the population with CKD (1).
It was observed that diabetic individuals were at a higher risk of developing sarcopenia, which corroborates the findings of Mori et al. (33), who evaluated 308 HD Japanese patients and used the criteria of the Asian Working Group for Sarcopenia for diagnosing sarcopenia. The mechanisms of how DM influences sarcopenia are not fully clarified, but a possible explanation may be related to the hormone insulin.
Protein degradation can be influenced by several factors, including a reduction in anabolic hormones such as insulin and an exaggerated production of reactive oxygen species (ROS) (34,35). Insulin seems to play a key role in the regulation of gene transcription of key proteins involved in the process of muscle protein degradation, particularly by suppressing genes related to skeletal muscle atrophy known as “atrogenes” (36).
Furthermore, it has been suggested that insulin resistance may be a response to protect the cell from damage caused by increased ROS activity, limiting the entry of nutrients into the cell. Thus, once insulin resistance is established in skeletal muscle, a vicious cycle of increased blood glucose levels can occur. This results in more oxidative stress and an ineffective increase in the concentration of circulating insulin, propagating the effects of catabolism in the muscle (37,38).
In this study, individuals with lower BMI were more likely to develop sarcopenia. Physical performance, muscle strength, and muscle mass are considered components of sarcopenia (1); thus, it is expected that these will be reduced among sick individuals. These findings are important in highlighting the role of muscle in the overall health of this population, since the reduction in lean mass has been associated with the risk of death, especially among individuals with BMI below 25 kg/m2. Such a condition suggests that body composition, especially when related to lean tissue, is more important than isolated BMI.
The evaluation of body composition showed that patients with increased % body fat had an increased risk of sarcopenia. This finding can be explained by the fact that obesity is characterized by increased production of fatty acids that can be stored in skeletal muscle, accumulating as intermuscular adipose tissue and intramyocellular lipids, and impairing biogenesis and mitochondrial function. Thus, dysfunctional mitochondria lead to greater production of ROS, resulting in apoptosis/autophagy of muscle cells, which is one of the potential mechanisms of obesity associated with sarcopenia (39).
Studies evaluating sarcopenia and inflammation in chronic renal patients undergoing HD are still scarce, and the understanding of confounding factors is still limited. In this sense, the present study presents positive aspects that should be highlighted, such as the use of DEXA as a reference method for estimating muscle mass, the use of a consensus for the definition of sarcopenia, and the levels of hsCRP for evaluating inflammation.
There are some limitations in this study. There is a bias inherent to the cross-sectional design; that is, reverse causality since exposure and outcome were evaluated simultaneously. Data regarding nutritional parameters (dietary intake) and physical exercise are lacking, which may have led to confusion regarding the decrease in muscle mass and diagnosis of sarcopenia in relation to inflammation; although the presence of diabetes has been assessed, no information is provided on the presence of comorbidities that also affect the inflammatory situation; sarcopenic obesity is invoked as a mechanism involved in the development of inflammation, but most of the patients have a BMI that does not correspond to obesity; and the small number of women with sarcopenia affects statistical significance and limits the extrapolation of the results.
In conclusion, in this study, the prevalence of sarcopenia as well as inflammation can be considered high. The presence of inflammation, DM, male gender, be older and a higher % body fat were risk factors for sarcopenia. BMI proved to be protective for sarcopenia, although it has been reported that the evaluation of body composition is more important than isolated BMI. Moreover, sarcopenic women seem to have a worse nutritional status when compared to those without sarcopenia. Men with sarcopenia are older, have increased inflammation, and worse nutritional status when compared to men without sarcopenia. Further studies are needed to investigate the relationship between sarcopenia and inflammation, seeking to better assess its impacts on HD patients in order to improve their nutritional status, and consequently to reduce the prevalence of sarcopenia and inflammation in this population.
Thus, further longitudinal studies are needed to evaluate and monitor patients undergoing hemodialysis treatment for inflammation and sarcopenia.














