INTRODUCTION
Disease-related malnutrition (DRM) represents an imbalance between energy and nutrient intake, and energy and nutrient requirements, leading to metabolic and functional changes that usually are hardly noticeable in early stages but can be assessed as changes in nutritional status and body composition (BC) markers during the course of disease (1). The Global Leadership Initiative on Malnutrition (GLIM) was convened in order to respond to the needs of the clinical nutrition and medical communities, with global reach, to focus on standardizing the clinical practice of DRM diagnosis. It is a two-step model. The assessment for diagnosis and DRM severity grading was based on five top-ranked criteria, including three phenotypic criteria (non-volitional weight loss, low body mass index, and reduced muscle mass) and two etiologic criteria (reduced food intake or assimilation, and inflammation or disease burden) (2).
These phenotypic and etiologic criteria can be useful in clinical practice to diagnose complex clinical situations such as cachexia and sarcopenia, and to support the management of these pathologies. As a result of the development of these criteria, the American Society for Parenteral and Enteral Nutrition (ASPEN) conducted a systematic review to validate BC methods (3). Morphological and functional criteria need to be evaluated within this classification for a better understanding of their clinical utility. There are other reference methods for BC assessment such as dual-photon X-ray absorptiometry (DXA) (4), but the technique is expensive and difficult. These limitations reduce its use in clinical practice.
Classic bioelectrical impedance analysis (BIA) estimates BC indirectly based on predictive equations, which limit its clinical use. Multi-frequency spectroscopic and segmental BIA devices open up a range of possibilities for other measurements such as monitoring body fluids. Vectorial BIA provides raw electrical values: impedance (Z), resistance (Rz), reactance (XC), and phase angle (PhA). Its direct application or vector representation have proven useful to assess BC changes in the short term, and act as a specific nutritional status marker, nutritional prognostic indicator, and morbidity and mortality risk indicator (5-7). In this sense, PhA provides a measurement for energy (electrical) changes, which is related to cell function and the composition of the internal environment, not from a molecular point of view but from a bioelectric one. Changes in cellular and tissue bioenergy are sensitive to nutritional and metabolic changes, and therefore provide comprehensive information about tissue composition and functionality.
The use of ultrasound for the morphological and structural study of muscle mass is to determine muscle architecture parameters such as muscle thickness, fascicle lengths, and pennation angles (8). Ultrasound has the advantage of being relatively affordable, is portable, and produces no ionizing radiation. More clinical research is needed to help establish evaluation patterns for ultrasound results that correlate with morbidity and mortality outcomes and other health indicators. We need to classify adipose tissue to complete BC assessment as the second component of fat mass (FM). In this sense, publications have been referenced with subcutaneous adipose tissue (SAT) at the level of the femoral area with muscle studies, and in the abdominal area with the possibility of evaluation of subcutaneous and visceral fat (9).
Classic laboratory data need to be adapted to more specific biomolecular markers that assess nutrition, inflammation (CRP/prealbumin), metabolic changes, etc. Functional assessment is always necessary not only for nutritional diagnosis but also to assess functional changes (10). Nowadays, estimated muscle strength using handgrip dynamometry should complement nutritional status assessments. Global assessments of body function remain to be systematized. Functional tests such as the Timed Up and Go (TUG) test, Gait Speed, or the Barthel Index (BI) should be included in nutritional assessments as they complement BC data (11).
It would be important, in this regard, to consider the incorporation of parameters for nutritional assessment that should be practical, sensitive, and specific, as well as reproducible in patient follow-up. In addition, these parameters should be convergent with other diagnostic tools for malnutrition, as well as capable of predicting clinical prognosis. We must provide a global view of all these techniques rather than the contribution of each one, that is, a morphofunctional assessment of DRM as a whole. The fundamental value of these techniques is their incorporation into routine clinical practice.
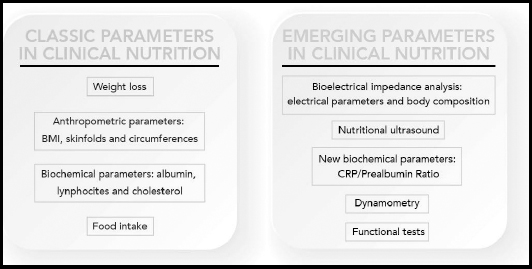
The most commonly used parameters of nutritional interest in clinical practice and their evolution are described in the figure below (Fig. 1). Some of them have been clearly established, classic nutritional parameters such as weight loss, BMI, skinfolds, circumference measurements, albumin, lymphocytes, cholesterol, and food intake, while other novel parameters for clinical nutrition are now emerging, and their introduction into clinical practice is generating an increasing amount of interest, such as BIA and PhA measurements, nutritional ultrasound, CRP/prealbumin, dynamometry, and functional tests.
Many of the classical parameters have a different diagnostic value in DRM. These parameters are included in most DRM screening tools and assessment tools (MUST, MNA, NRS-2002, SGA, etc.). For example, albumin is a global marker of morbidity and mortality that correlates with disease severity but loses specificity when considered of purely nutritional value. On the other hand, intake assessment with sensitive techniques applicable to clinical practice is a fundamental factor in the detection of DRM, and must be understood as an essential part of the diagnostic criteria (GLIM CRITERIA) (2).
The aim of this publication is a narrative review of all the potentially useful parameters for nutritional assessment, with a practical contextualization of commonly used tools in clinical practice, and an assessment of present and future application options.
MORPHOFUNCTIONAL PARAMETERS IN CLINICAL NUTRITION
The definition, usefulness, and limitations of the most commonly used parameters of nutritional interest in clinical practice are described throughout the article.
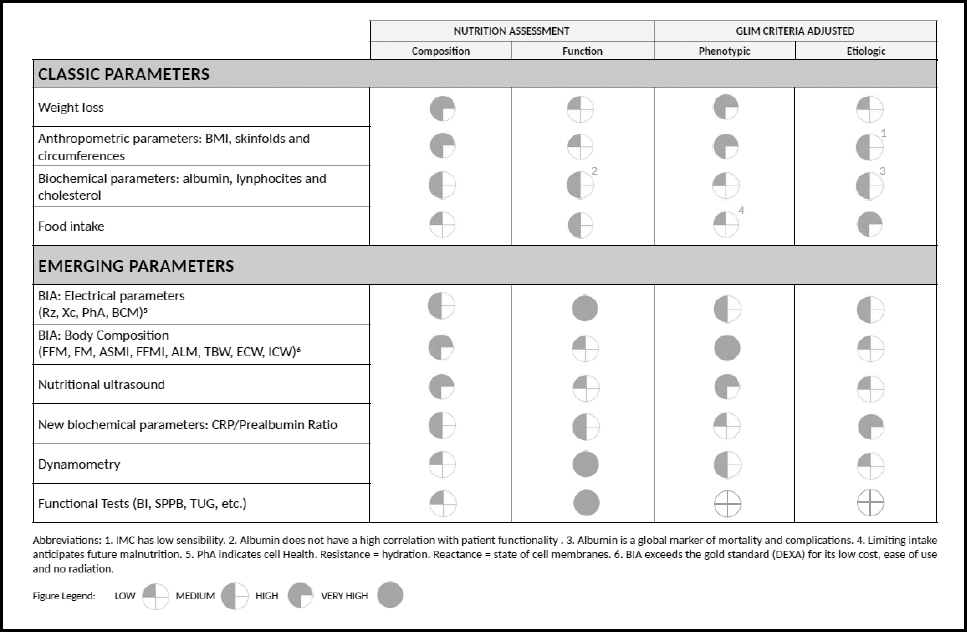
These morphofunctional parameters in the diagnosis of DRM could be applied to the recent GLIM criteria, contributing to measure some different etiologic and phenotypic criteria as described in figure 2.
CLASSIC PARAMETERS IN CLINICAL NUTRITION
WEIGHT LOSS
Weight is the simplest method, and is important, to assess nutritional status but is not sensitive enough for the early detection of DRM (12). The clinical use of weight loss is important when screening patients for risk of DRM, as well as for DRM diagnosis and to estimate nutritional requirements (13). According to the GLIM criteria, losing more than 5 % of body weight within 6 months, or more than 10 % beyond 6 months (2), is one of the phenotypic criteria used to diagnose DRM.
There are several potential limitations such as: lack of information about usual weight, presence of oedemas, or other alterations in hydration status (12,14). Involuntary weight loss is a key parameter for nutritional assessment as it indicates a negative energy balance (15). It should be a required value in a patient's medical record.
ANTHROPOMETRIC PARAMETERS: BODY MASS INDEX, SKINFOLDS, AND CIRCUMFERENCES
Body mass index (BMI) (16) is calculated by taking a patient's weight in kilograms and dividing it by their height squared in meters (BMI = weight [kg] / height [m2]).
BMI is an anthropometric measure used for defining DRM cut-off points, using BMI values < 20 kg/m² for people under 70 and < 22 kg/m² for patients over 70 years of age (17). Based on the GLIM criteria, severe DRM corresponds to BMI values < 18.5 kg/m² in people under 70 and < 20 kg/m² in patients over 70 years of age (2).
BMI has low sensitivity for the early detection of DRM (21 % sensitivity and 95 % specificity) (12). Furthermore, it has been observed that catabolic patients may lose more than 10 % of their weight over 3-6 months and have BMI values above the normal range (17). It is a required parameter in nutritional assessment, but it is not an exclusion criterion for diagnosing DRM (18).
Skinfolds and muscular circumferences are useful at the individual level but are limited due to the difficulties inherent in extrapolating a clinical result and its variability during short-term follow-up. They provide a measure of the depletion or excess of adipose tissue and the muscular protein compartment, but indirectly and always in relation to BMI (12,19).
BIOCHEMICAL PARAMETERS: ALBUMIN, LYMPHOCYTES, AND CHOLESTEROL
Biochemical parameters such as albumin, lymphocytes, and cholesterol are general health markers that provide indirect information about nutritional status due to their correlation with whole-body protein, energy status, or nutrient balance. Albumin, the main visceral protein, is the most established marker because of its high correlation with morbidity and mortality in different clinical situations (20,21). These biomarkers are exposed to many interferences from inflammatory processes since many of them behave like acute phase reactants in them (21).
Total lymphocyte count and low cholesterol levels, which are usually included in automated screening methods, show an indirect correlation with energy restriction and are included in different DRM diagnostic and coding systems (22,23). However, they are not included in the GLIM criteria for the diagnosis of DRM (2).
FOOD INTAKE
An insufficient nutrient intake is an etiologic factor for DRM during illness due to different factors, as reported in the GLIM criteria (2). Food intake is determined with the use of categories to measure the amount of food eaten by patients during a period of time. Quartiles of intake is the most frequent semi-quantitative method used in different diagnostic test approaches, and is useful when setting therapeutic guidelines for the nutritional support required (24,25). Food intake assessment methods require collaboration by patients.
Lack of intake is the presumption of future DRM. Anorexia is one of the main symptoms associated with disease. This factor and increased requirements due to the inflammatory process cause intake restriction, which can lead to DRM.
EMERGING PARAMETERS IN CLINICAL NUTRITION
There are emerging techniques that provide information in the analysis of BC and functionality such as BIA and PhA measurements, nutritional ultrasound, CRP/ prealbumin, dynamometry, and functional tests. These techniques are accessible to routine clinical practice. On the other hand, there are other standardized techniques to measure BC that are focused on research studies such as DXA and Computed Tomography (CT). These emerging techniques are exploration techniques that can be applied as many times as needed to assess nutritional status, are not invasive, and do not pose any risks to patients from ionizing radiation, which does not imply that we can use DXA and CT techniques whenever they are available. The difference is that these emerging techniques cannot be planned prospectively for the clinical course of patients in clinical nutrition.
BIOELECTRICAL IMPEDANCE ANALYSIS
BIA is a classic BC technique. However, for years the direct bioelectric data that avoid the bias and errors introduced by formulas and equations have not been studied in depth. In recent years, the analysis of bioelectric data has been re-emerging due to its correlation with prognostic factors and its non-dependence on predictive formulas.
BIA results can be divided into BC electrical parameters and laboratory indices. Each one of these areas focuses on different aspects of BC and function. Bioelectric parameters are raw measures obtained with the direct measurement without interference of anthropometric factors such as weight or age. BC parameters are based on the application of predictive equations for the calculation of the different fat free mass (FFM) and FM compartments, adjusted for clinical variables. This point is important due to the possibility of error and inaccuracies derived from the use of predictive formulas or their application in a specific clinical area.
The global and muscular indices obtained by recalculation of bioelectrical and clinical parameters contribute more information to the clinical course of specific aspects such as muscle structure and function, or the degree of hydration or nutrition of the body. Different formulas determine varying results between measurements, with a possible interference of anthropometric and clinical factors.
This emerging technique has a number of limitations that are inherent in the measurement technique and the characteristics of the different devices with their bioelectric measurement plates. The most important biases also affect measurement protocols with the possibility of intra- and inter-observer bias. They are not widely used techniques in nutrition units due to the limited number of normal population references (percentile tables by age and sex) and of individualized cut-off points for each pathology in order to enable clinical decision-making (3).
BIA and body composition
BIA is an indirect method to measure BC, based on the human body's ability to conduct electricity. The current is transmitted through liquids and electrolytes, while fat and bone are not really conductors (26,27). Through raw impedance parameters, such as Rz and Xc, the PhA can be obtained (PhA = arc tangent (Xc / R) x 180 ° / π). By definition, PhA is positively associated with tissue reactance (associated with cell mass, integrity, function, and composition) and negatively associated with resistance, which depends mainly on the degree of tissue hydration (26,27) (Fig. 3).
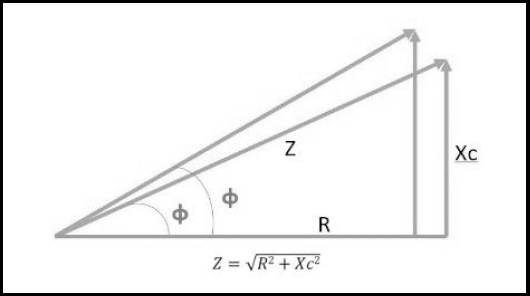
Figure 3. Graphical representation of impedance components: Xc: reactance; Z: impedance; Rz: resistance; φ: phase angle. Source: own elaboration.
At present, BIA is probably the most widely used method to study BC in different contexts, mainly because of its low cost, because it is easy to use and transport, and because inter-observer variability is lower than with other techniques (6,25). PhA is considered a good indicator of cell integrity and has been proposed as a nutritional status marker for adults and children after having been studied in the setting of numerous pathologies. It has also been proposed as a useful prognostic marker for several clinical conditions. Several authors also suggest that it may be a useful tool to evaluate the progression of disease. In general, lower levels of PhA suggest a worse prognosis and greater morbidity and mortality (28,29).
In healthy populations, PhA varies physiologically depending on sex, age, BMI, and race. Several authors have studied the reference values for PhA according to variables in healthy populations, and they are between 5.5 and 9 ° (30). At present, the absence of reference values for the Spanish healthy population and for different pathologies limit its use in everyday clinical practice. It is important to follow the protocol for measuring and positioning the patient.
BIA AND BODY COMPOSITION
There are different aspects in the analysis of BC by BIA. There is the analysis of water and BC and, on the other hand, the measurements and muscle indexes that are of great interest in the GLIM criteria (2).
BIA BC data provide information about the different body compartments (FFM and FM) and also about hydration: total body water (TBW), extracellular water (ECW), and intracellular water (ICW). We can also obtain direct muscle data such as Appendicular Skeletal Muscle Index (ASMI), Fat Free Mass Index (FFMI), and Appendicular Lean Mass (ALM). All of these parameters have are high-value because they focus on the distribution of body compartments with great relevance in nutritional assessment. GLIM recommended cut-off values for muscle mass reductions from the European Working Group on Sarcopenia in Older People (EWGSOP), and from The Foundation of National Institute of Health (FNIH) initiative and the Asian Working Group on Sarcopenia (AWGS) (2).
The global indices provide information about different aspects as adapted to clinical practice.
NUTRITIONAL ULTRASOUND
The use of ultrasound is an emerging technique that is based on the application of ultrasound to determine the surface of muscle tissue. Particularly, ultrasound analysis allows us to measure key muscle architecture parameters such as muscle thickness, fascicle length, and pennation angles (8).
Muscle ultrasound techniques
Most studies focus their attention on the rectus femoris muscle of the quadriceps or on combinations of muscle groups that involve major muscle bundles with an important functional role on the patient's gait or basic activities of daily living (BADLs). Measurement of the rectus femoris muscle of the quadriceps is one of the most widely used approaches because of its correlation with force and functional execution or performance tests (31).
Currently, all definitions of DRM include its effect on muscle mass measurements; however, there is not just one assessment method. Classic imaging techniques such as DXA, computed tomography, and magnetic resonance imaging are considered "gold standards" but their clinical application is difficult under routine conditions (8,32).
Adipose tissue ultrasound techniques
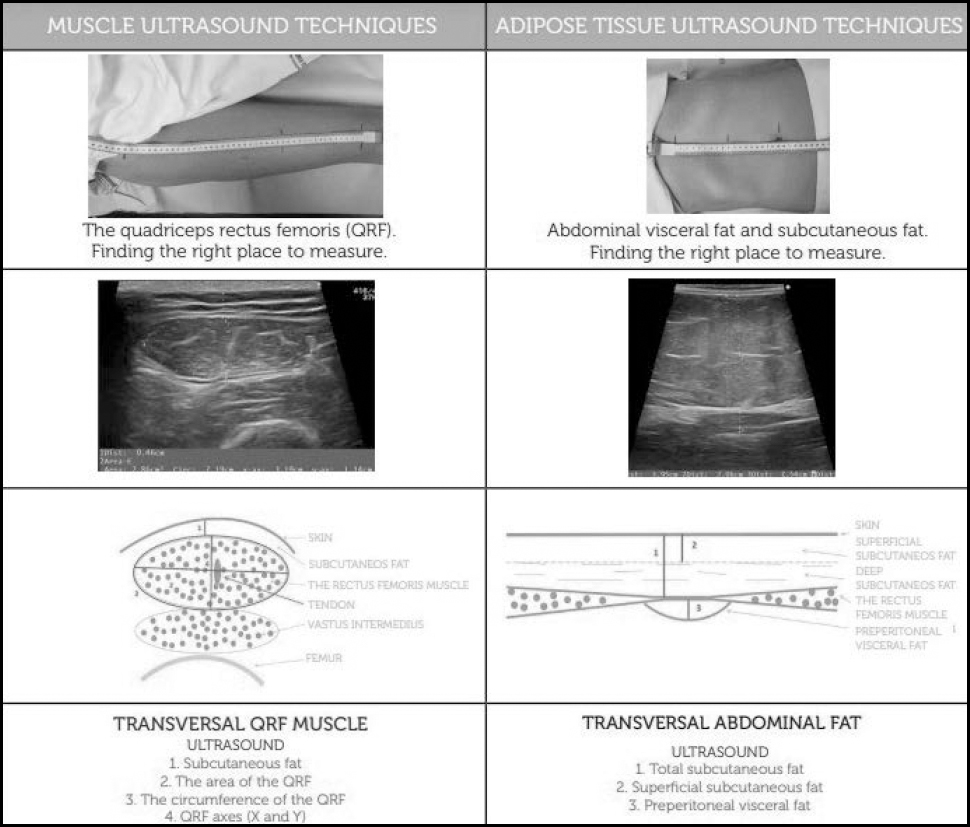
Ultrasound techniques to assess adipose tissue (FM compartment) in clinical nutrition evaluate subcutaneous (superficial and deep-layer) and visceral adipose tissues (Hamagawa's technique) (9). Figure 4 describes the available nutritional ultrasound techniques.
The clinical utility of this technique is to assess fat distribution and correlate this with clinical variables. Each type of adipose tissue may be related to different functions. The superficial subcutaneous fat layer is related to energy reserve. The deep subcutaneous fat layer may play a role in neuroendocrine regulation via the secretion of adipokines (adiponectin). The preperitoneal visceral adipose tissue is a visceral ectopic tissue, the equivalent of hepatic steatosis and other pathological adipose tissues. The clinical value of visceral deposits is related to metabolic manifestations such as diabetes or atherosclerosis.
Ultrasound has the advantage of being relatively affordable and portable, and produces no ionizing radiation. Several studies have confirmed the reliability of this technique to measure the size of the quadriceps muscle in a healthy population. Studies have been published on the reliability of rectus femoris ultrasound measurements with an intraclass correlation coefficient (ICC) of 0.97 (95 % CI: 0.92-0.99) for test-retest reliability of this technique (33). A femur muscle area below 5.2 is associated with frailty, which involves a worse survival prognosis (34). There are also studies on the application of ultrasound measurements in clinical practice for the nutritional assessment of different pathologies, for example in critical patients (35) and the elderly (36).
The main limitation is the uniform application of this measurement in clinical practice, or the recommendations for evaluating nutritional status. More clinical research is needed to help establish evaluation patterns for ultrasound results that correlate with morbidity and mortality outcomes and other health indicators.
There are other morphofunctional techniques such as CT, magnetic resonance imaging, and DXA that have been shown in multiple studies to have high precision and reliability (37), and provide diagnostic values for the evaluation of muscle mass and sarcopenia with an association with morbidity and mortality (38), especially in cancer patients, since they provide images that are used for the planning of radiotherapy treatment (39); however, they have the limitation of poor applicability in clinical practice because of limitations such as exposure to ionizing radiation, and need for specific skills and knowledge of anatomy to interpret results, in addition to limited availability and high cost. The objective of this narrative review is to analyze DRM screening and diagnostic techniques that can be implemented in routine clinical practice.
BIOCHEMICAL PARAMETERS: CRP/PREALBUMIN RATIO
Prealbumin, a protein that transports thyroxine, is much more sensitive to any changes in whole-body protein status than albumin and transferrin, because it has a very short half-life (2-3 days). Unlike albumin, hydration status does not affect prealbumin (40). Its association with ultra-sensitive C-reactive protein (CRP) levels, a pure marker for inflammation in the body, may increase its interest as a predictor of morbidity and mortality, and of nutritional/inflammatory changes (21,41).
Prealbumin is considered by some scientific societies the best laboratory protein parameter to monitor nutritional status and its therapeutic changes (13). As with the rest of plasma proteins, infections and other acute inflammation processes can interfere with its results (42). In critical patients a cut-off point of > 0.24 for the CRP/prealbumin ratio (CRP mg/dL / prealbumin mg/dL) has been found to be a predictor of mortality and of hospital stay extension (43,44).
More prospective studies are needed to provide cut-off points, involving therapeutic approaches with clinical benefits for patients.
DYNAMOMETRY
Dynamometry is a functional method to assess muscle strength that measures handgrip strength. It is easy and quick to carry out. It has good reproducibility and a high sensitivity and specificity for the prediction of post-surgical complications, stay in hospital, higher rate of readmissions, and a decline in physical condition (12,45,46).
Dynamometry has become a general marker for nutritional status and is being used as a variable result in nutritional interventional studies (46). This parameter is very sensitive to re-nutrition changes, which is why it is very useful when monitoring the effects of nutritional therapy even in the short or medium term (12).
Jamar® dynamometers are most widely used in international studies, and have several handle positions. Measurements obtained must be compared with population average values according to age and sex in tables. Sánchez et al. have published, in their epidemiological study "Pizarra", the reference values for dominant hand muscle strength for this type of dynamometer in a Spanish population to assess DRM. Apart from this, they concluded that hand grip dynamometry is associated with lean mass (LM), which confirms its usefulness in nutritional assessment (47).
It should be noted that hand grip strength measurements are an indicator of upper limb strength and, even if they have predictive potential, they should not replace the evaluation of activities of daily living (ADLs), the strength of lower limbs, or gait speed in fragile populations such as the elderly or patients suffering from neuromuscular disorders (46).
There are some limitations related to hand grip measurements; for example, there are no measurement protocols. Another limitation is that they require the collaboration of the patient (45). However, key application scenarios are still to be defined for nutritional screenings as a diagnostic measurement or for monitoring nutritional recovery.
FUNCTIONAL TESTS
Functionality can be measured through self-reported scales or rating scales based on the reports of others, or through objective performance or execution tests. Basic activities of daily living (BADLs) include self-care, mobility, and transfer activities, which are mainly necessary to maintain independence at home (48).
The BI (also known as The Maryland Disability Index) is one of the best scales to assess BADLs (49). Studies have shown that patients at risk of DRM or with DRM show a decline in ability to perform BADLs, resulting in greater functional dependence (50). BI provides measurements that are easy to apply and interpret with a high degree of reliability and validity. In addition, it can be adapted to different cultural environments, and is useful to monitor the progress of patient functionality (49).
TUG test measures the time a patient takes to rise from a chair, walk 3 meters, turn, walk back, and sit down again (51). This is a quick test, easily included in clinical practice, that requires no special training for the staff in charge, provides good inter-observer and intra-observer reliability, has adequate validity, and can predict fall risks with a sensitivity and specificity higher than 80 % (48).
A functional diagnosis of patients is important for DRM. This should include evaluating their activity and monitoring any clinical changes over time in order to implement therapies to improve balance, stability, and gait to reduce dependence and/or disability. In the end, the ultimate purpose is the patient's functional recovery, with positive changes in weight, FM, FFM, and functionality.
CONCLUSIONS AND FUTURE LINES OF RESEARCH IN THE FIELD OF CLINICAL NUTRITION
In clinical nutrition, due to the absence of universally accepted criteria to define DRM based on standard parameters that may be applied to clinical practice, it has become essential to establish lines of research to provide results that help implement a clinically accurate approach, based on final health outcomes. The loss of LM affects the clinical outcome of both acute and chronic illnesses, and as such, its assessment is of particular interest in clinical nutrition. The application of emerging techniques such as BIA and nutritional ultrasound is becoming increasingly important for nutritional status assessment.
Regarding BIA, despite its being an indirect method and having limitations in obese patients with BMI > 35 kg/m2 and other populations, evolution towards multifrequency devices, the introduction of specific software, and most particularly the assessment of raw parameters such as PhA allow a direct approach to cell function and health status measured as body cell mass (BCM) and both intracellular and extracellular hydration status (52). Considering PhA, there are at least 19 clinical trials in subjects with different pathologies: cancer patients, critically-ill patients, elderly patients, patients with amyloidosis, etc., which explore the future application of this parameter for studying and monitoring patients in clinical practice (see at https://clinicaltrials.gov/; condition or disease: phase angle).
It is necessary to perform a standardization of imaging techniques such as nutritional ultrasound and CT to establish reference values and critical points for decision-making. On the other hand, more prospective studies are needed to assess its prognostic value in the field of nutritional intervention for DRM in order to evaluate response times and to describe the minimum clinically significant change in all these parameters. These techniques must be correlated with other classical parameters of nutritional assessment already established that provide nutritional prognostic value (e.g., subjective global assessment, SGA).
Functional changes in clinical nutrition must be studied in depth as they are closely related to health outcomes. Some studies are being conducted regarding hand dynamometry, TUG testing. and other functional tests to evaluate the role nutritional intervention plays in a patient's functional recovery.
In the future, specific biochemical markers for muscle proteins, adipose tissue, and different metabolic pathways that provide information on DRM and inflammation status shall be equally validated. It is necessary to develop an expert positioning on the applicability of these techniques and their incorporation into routine clinical practice.