INTRODUCTION
In recent years, the need to improve the quality and efficiency of health systems has led to a growing interest in the application of various tools for achieving a better management. Quality indicators that express the extent of achievement for key objectives in organizations stand out among these instruments. In general terms, these indicators focus on specific quality dimensions (accessibility, patient satisfaction, health outcomes, safety, etc.). They are intended to meet key requirements such as relevance, feasibility, and reliability, and meant to be based on evidence (1).
In order to achieve correct interpretations, the results of the indicators should be compared with reference standards that indicate the limit beyond which the levels of compliance can be considered adequate. This comparison allows determining corrective measures to improve outcomes in organizations. The standards are intended for use in practice. When they are defined, not only the level of compliance that is convenient from a theoretical point of view should be taken into account, but also the degree of real difficulty they pose. In this sense, there are many factors that can promote compliance with quality indicators. These factors may depend on: the organizations (management involvement, leadership style, institutional culture of quality improvement, and availability of resources); the professionals (awareness and recognition of recommendations in clinical guidelines, time restrictions, previous experiences); or the activity being monitored (coherence and strength of scientific evidence, associated technical complexity) (2).
In Spain, quality indicators have been proposed for assessing the various stages of clinical nutrition care (3,4). The reference standards for these indicators are mostly theoretical values obtained from arbitrary criteria or from research studies. Therefore, the goal of the present study was to obtain sufficient information to determine standards, based on daily practice, for relevant and feasible indicators in routine application.
MATERIALS AND METHODS
The present multicentre cross-sectional study was conducted between 2018 and 2019. First, a questionnaire was prepared and sent to the members of the Spanish Society of Clinical Nutrition and Metabolism (SENPE) by email. Subsequently, the responses included in the study were those sent by health professionals that belonged to SENPE and were performing their professional activity in the field of clinical nutrition, either in public or private hospitals nationwide.
The questionnaire included eight quality criteria (29 items in total) extracted from the document "Process of Clinical Nutrition. Self-assessment guide" prepared by the SENPE Management working group. It was based on the relevance and feasibility criteria for indicators used in nutrition units that were obtained in 2012 (5).
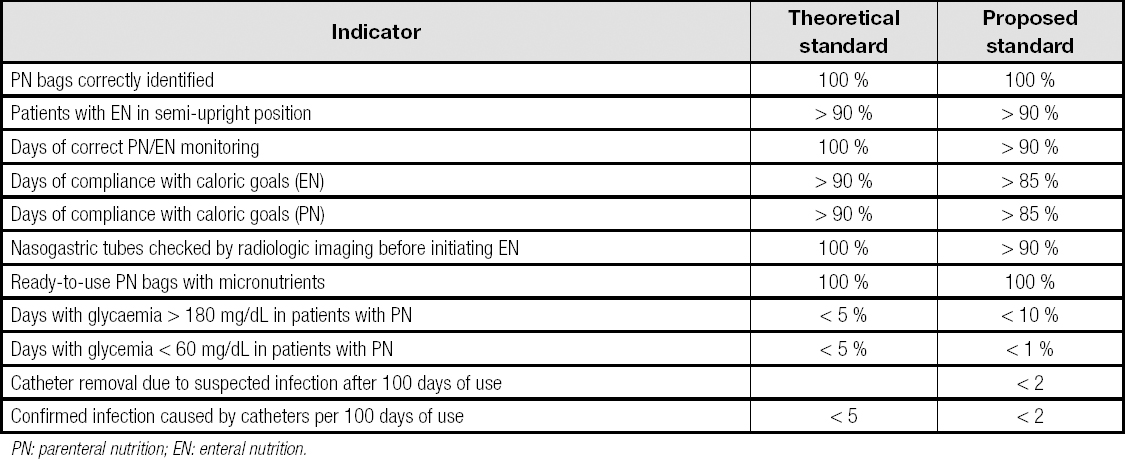
Table I shows the quality indicators included in the survey. The level of compliance with each indicator (process completed/process assessed x 100) was compared with the theoretical standard proposed by the existing literature (theoretical standard). Based on this result, but also taking into account the relevance of compliance with indicators and the difficulty they entail, standards were proposed for each indicator (proposed standard).
RESULTS
Responses were obtained from 15 health centers, of which: one had less than 200 beds (6.7 %); three had 201 to 500 beds (20.0 %); six had 501 to 1,000 beds (40.0 %); and five had more than 1,000 beds (33.3 %). Nutrition units or multidisciplinary nutritional support teams were responsible for nutritional support in 13 of the centers (86.7 %). In the other two centers nutritional support was in the hands of professionals not organized in a functional unit.
IDENTIFICATION OF PARENTERAL NUTRITION BAGS
Data were obtained from 12 centers. The assessment included 2,380 parenteral nutrition bags, which meant an average of 198.5 (330.5) bags per center assessed (minimum 4 bags/center, maximum 1,101 bags/center). Of these bags, 2,374 had an identifying label. This fact indicated a degree of compliance of 99.7 % (theoretical standard = 100 %).
SEMI-UPRIGHT POSITION OF PATIENTS WITH ENTERAL NUTRITION BY NASOGASTRIC TUBE
Data were obtained from 12 centers, with a total of 620 patients (mean, 57.7 [82.4] patients/center; minimum 6 patients/center, maximum 278 patients/center). The degree of compliance was 96.8 % (theoretical standard > 90 %).
CORRECT MONITORING OF NUTRITIONAL SUPPORT
This item was answered by 13 centers. A total of 1,050 visits to patients were analyzed, corresponding approximately to 80.7 (107.8) visits per center (minimum 9, maximum 400), of which 988 were considered correct according to the definition of the criterion. Compliance with the standard was 94.1 % (theoretical standard = 100 %).
MEETING THE CALORIC GOAL WITH ENTERAL NUTRITION
Data from seven centers were analyzed, with a total of 429 days of enteral nutrition (61.3 [54.4] days/center; minimum 15 days/center, maximum 137 days/center).
Caloric requirements were met in 369 days, which represented a standard of 86.0 % (theoretical standard > 90 %).
MEETING THE CALORIC GOAL WITH PARENTERAL NUTRITION
Six centers submitted data for this criterion, including 380 days of parenteral nutrition (63.3 [50.8] days/center; minimum 12 days/center, maximum 148 days/center). Caloric requirements were met by administering parenteral nutrition for 335 days (88.2 %) (theoretical standard > 90 %).
CHECKING THE PLACEMENT OF ENTERAL FEEDING TUBES
Eleven centers answered this item, with a total of 218 patients and a mean of 19.8 (18.2) patients per center (minimum 5 patients/center, maximum 61 patients/center). Correct probe checking had been performed in 188 patients, which represented a standard of 86.2 % (theoretical standard = 100 %).
MICRONUTRIENT SUPPLY IN ‘READY-TO-USE' PARENTERAL NUTRITION BAGS
These data were only obtained from four hospitals, and a total of 160 bags of parenteral nutrition were assessed (40.0 [72.0] bags/center; minimum 2 bags/center, maximum 148 bags/center). Micronutrients had been added to 158 bags (98.8 %) (theoretical standard = 100 %).
GLYCEMIC CONTROL IN PATIENTS WITH PARENTERAL NUTRITION
Twelve centers sent data related to glycemic control. A total of 3,782 days with parenteral nutrition could be assessed, with 315.2 (424.2) days/center (minimum 5 days/center, maximum 1,226 days/center). Hyperglycemia (> 180 mg/dL) was observed during 595 days (15.7 %), and hypoglycemia (< 60 mg/dL) during 18 days of follow-up (0.5 %) (theoretical standard = 5 % in both cases).
INFECTION OF PARENTERAL NUTRITION CATHETERS
Data from six hospitals were analyzed. The results are shown in table II. Table III shows the theoretical and proposed standards for the most relevant indicators of a hospital's artificial nutritional support process.
Table II. Parenteral nutrition catheter-related infection
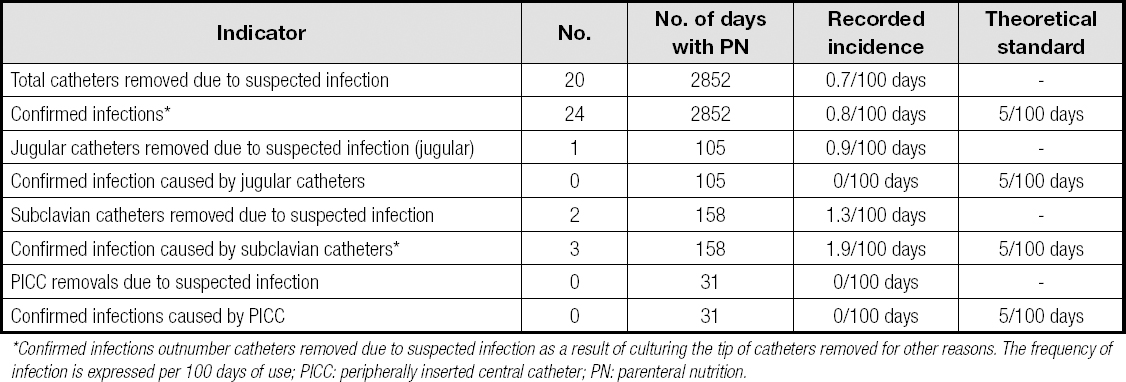
*Confirmed infections outnumber catheters removed due to suspected infection as a result of culturing the tip of catheters removed for other reasons. The frequency of infection is expressed per 100 days of use; PICC: peripherally inserted central catheter; PN: parenteral nutrition.
DISCUSSION
In the last decade, quality management has been progressively established in health systems. This fact promoted important changes in their organization. These changes have directly affected clinicians, whose objectives have gone from providing healthcare based on the best available scientific evidence to also incorporating the satisfaction of different stakeholders (patients, relatives, managers, providers, healthcare teams, and society). This way, in recent years, both scientific societies and health agencies have created indicators for controlling healthcare quality.
In conjunction with the Spanish Society of Hospital Pharmacy (SEFH), the Spanish Society of Clinical Nutrition and Metabolism (SENPE) has developed the "Guidelines for the assessment of the clinical nutrition process", which discusses the sub-processes involved in nutritional support for hospitalized patients (3). Each sub-process is accompanied by one or more quality criteria, with their definition, indicators, and theoretical standards.
The goal of the present study was to propose standards based on clinical practice, applying those quality indicators considered most relevant and feasible (5). Responses were obtained from 15 centers distributed throughout the different Spanish autonomous communities, 11 of which had more than 500 beds and a nutrition unit. This fact indicates that the data presented can be considered representative of the nutritional care provided by medium/large centers with structured and recognized units for nutritional support (2).
Among the results obtained, the high degree of compliance with some of the indicators stood out, namely: correct identification of parenteral nutrition bags; semi-upright position of patients with enteral nutrition; administration of micronutrients in ready-to-use parenteral nutrition bags; and days of glycemia below 60 mg/dL. Use of protocols and systematization of activities, mainly in hospital pharmacies, may have contributed to these results.
On the other hand, compliance with other indicators was slightly lower. Examples are: correct monitoring of artificial nutrition; radiographic assessment of the correct position of feeding tubes; and compliance with caloric goals in parenteral and enteral nutrition. Therefore, it is considered advisable to propose a lowering of the standard because, possibly, the underlying difficulties were fundamentally due to the workloads for the case of monitoring. The interruption of support may have occurred due to various complications and diagnostic or therapeutic procedures in the case of compliance with caloric goals. Radiographic probe testing should be performed due to frequent pull-outs that can make further compliance difficult in some cases.
Regarding the control of hyperglycemia, the proposed standard was raised to 10 % of days instead of the 5 % theoretically proposed. During the administration of parenteral nutrition, 50 % of patients exhibited some elevated glycemia values, especially those with previous diabetes, greater intravenous blood glucose supply, hyperglycemic drugs, or infectious complications (6). Hyperglycemia during parenteral nutrition is an independent factor for in-hospital mortality, hence the relevance of its control (7). The control of hyperglycemia is counterbalanced by the risk of developing hypoglycemia. The latter has been addressed by studies in up to 7 % of patients with parenteral nutrition, and is also a risk factor for mortality in hospitalized patients (8,9). The lower degree of hyperglycemia control with respect to hypoglycemia may have resulted from a conservative use of hypoglycemic treatment, in addition to the difficulty in controlling it due to clinical factors and hyperglycemic drugs.
Regarding the infection rate of the central venous catheters used for administering parenteral nutrition, multiple data are available on the rate of complications of these catheters in home parenteral nutrition. In Spain, the most frequent complication in patients with home parenteral nutrition has been infection, with a rate of 0.64 infections per 1,000 days of central venous catheter use (10). In hospitalized patients, the reported infection rate has been highly variable, between 0 and 4.9 infections per 1,000 days (11-13). Variability may depend on factors such as the type of central venous catheter, local catheter management protocols, underlying diseases, or the definition of infection associated with the catheter used. According to the data found in the present study, infection rates have been lower than reported in other studies, which could be due to the lower number of hospitals that provided data for this indicator, their having implemented infection control programs, or a different recording method. Even so, the standard has been significantly lowered in the present proposal.
The strengths of the present study include the number of centers that answered the survey and, above all, the number of patients included. Among its limitations, the fact stands out that responses were mainly obtained from larger hospitals that had nutrition units among their healthcare services. However, the study did not provide enough data about nutritional support in smaller hospitals without a defined structure for the provision of nutritional support, nor were data recorded for all the indicators analyzed in all hospitals.
In conclusion, the data obtained in the present study suggest that nutrition units in Spain perform their activity with a high quality degree. In addition, this work promotes the creation of initiatives to assess the quality of nutritional support in Spain, providing quality standards based on actual data. An immediate objective for the future should be obtaining information from the maximum number of centers possible, determining which are the best ones, learning from them, and ultimately benchmarking.