INTRODUCTION
Approximately 1 out of 11 adults worldwide have diabetes mellitus, with 90 % of cases corresponding to diabetes mellitus type 2 (DM2) (1). This incidence has increased by 4.8 % annually between 2002 and 2012 (2). The beginning of diabetes mellitus often occurs within years before diagnosis; worldwide, it was estimated that 45.8 % (or 174.8 million) of DM2 cases in adults were undiagnosed (3). Approximately 12 % of global health expenditure was estimated to be devoted to diabetes in 2015 (4). Besides genetics, obesity is probably the most important trigger factor for DM2. The “Milan Declaration 2015” by the European Association for the Study of Obesity (EASO) has defined obesity as a “progressive disease” that has its onset in being overweight, and has declared it to be the main “gateway” to many other diseases such as most non-communicable diseases (NCDs). The central role of obesity in diabetes, hyperlipidemia, and hypertension is recognized, with the consequence of increased cardiovascular morbidity and mortality (5).
It has been shown that body weight itself is a poor measure of corporal body fat. Almost 100 % of people with body mass index (BMI) > 30 kg/m2 have a high percentage of fat, just like a third of people with normal weight (6). BMI is a diagnostic parameter for obesity but does not reflect fat content, since people vary in their musculature and degree of sarcopenia, which increases or decreases their weight thus altering the fat-muscle ratio (5,7).
However, even if BMI was a perfect measure of body fat content, the limits for defining obesity and the selection criteria for bariatric or metabolic surgery are completely arbitrary. On the other hand, the distribution of body fat is important since visceral fat confers cardiometabolic risk and gynecoid fat even has a cardiovascular protective effect. Ectopic fat (liver, pericardiac, intramuscular) also confers metabolic risk and, in addition, the inflammatory state of adipose tissue is associated with insulin resistance (7).
The goal of DM2 treatment is to achieve glycemic objectives individually according to life expectancy and patient preferences.
Most of the patients with diabetes have complicated glycemic control that worsens over time. Increasing the dose of an existing oral agent is generally the first step for maintaining control but results are limited. Therefore, patients often require the addition of 1 or more oral agents, some of which - as we know - increase the baseline weight of patients (8).
In 2007 the delegates at the 1st Diabetes Surgery Summit (DSS-I), an international consensus conference, reviewed the available clinical and mechanistic evidence and recommended expanding the use and study of gastrointestinal surgery to treat diabetes, even in individuals with only mild obesity. In the ensuing years, the concept of “metabolic surgery” or “diabetes surgery” has become widely recognized in academic circles, and accordingly, most major worldwide bariatric surgery societies have changed their names to include the word “metabolic” (9).
There is an increase of references in the literature that justifies the inclusion of metabolic surgery in the treatment algorithms for patients with obesity and DM2. These techniques have traditionally been known as “bariatric surgery,” which denotes surgical procedures meant to induce weight loss (10). However, several studies have demonstrated that these techniques improve metabolic derangements independently of weight loss. These surgical procedures alter the gastrointestinal hormones that play an important role in glucose homeostasis (11).
Metabolic surgery is a vastly underutilized tool for the treatment and/or cure of DM2, which has led to recognize the need to inform diabetes care providers about the benefits and limitations of metabolic surgery. The 2nd Diabetes Surgery Summit (DSS-II) was convened in collaboration with six leading international diabetes organizations: the American Diabetes Association, International Diabetes Federation, Chinese Diabetes Society, Diabetes India, European Association for the Study of Diabetes, and Diabetes UK. The overarching aim of this consensus conference was to review the available evidence and to develop global recommendations that integrate medical and surgical therapies in a rational treatment algorithm for DM2 (9).
Recent efforts have expanded the indications of metabolic surgery. It is reflected in the DSS-II, which includes patients with a BMI of 30.0 to 34.9 kg/m2 with uncontrolled DM2 despite optimal pharmacological treatment. The BMI threshold is reduced by 2,5 kg/m2 for Asian patients because they typically have more visceral fat compared to Caucasians with diabetes and the same BMI (9,11).
One-anastomosis gastric bypass (OAGB) is recognized by the IFSO (International Federation of Obesity Surgery and Metabolic Disorders) as a bariatric/metabolic procedure not to be considered for research; however, it is not recommended in diabetic non-obese patients or for patients with class-1 obesity (12), since in the DSS-II the only four accepted surgical approaches for weight loss and DM2 remission are; biliopancreatic diversion, Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy, and adjustable gastric band. These are listed by gradient from highest to lowest in terms of efficacy, and inversely proportional to the comparative safety of these procedures (9).
MATERIAL AND METHODS
A retrospective review was carried out between 2016 and 2019 of all patients with DM2, BMI between 25 and 29.9 kg/m2, and serum C-peptide level higher than 1 ng/dL who underwent laparoscopic one-anastomosis gastric bypass (OAGB) as metabolic procedure. A modification of the original technique of Dr. Miguel Ángel Carbajo Caballero was made by performing the anastomosis with a ratio of 20/80 of the total length of the small intestine.
All patients had a 24-month follow-up, with measurements of fasting serum glucose, HbA1c, and BMI at 3, 6, 12, and 24 months after surgery. All cases were operated upon by a single bariatric surgeon in the same private institution.
The series did not include revision surgery cases; patients who had already lost weight but without DM2 remission with another surgical technique, and patients with gastroesophageal reflux documented by upper gastrointestinal endoscopy were also excluded, as well as patients with BMI > 30 kg/m2, prior gastric surgery, inflammatory bowel disease, major psychiatric disorders, and alcohol or drug abuse.
Continuous variables were expressed as means. All patients included were informed of the risks and benefits of the procedure, and underwent surgery after signing an informed consent.
Surgical technique: 6 trocars were used for the procedure - a 10-mm one for the optical port, two 12-mm trocars for the staplers, and three 5-mm trocars for the liver retractor and the first assistant.
The first step of the procedure was to identify the ligament of Treitz in order to measure total intestine length and choose the anastomosis loop site. Then the stomach's angle of His was released by sectioning the phrenoesophageal ligament.
Afterwards, with an ultrasonic device, the blood vessels of the lesser curvature of the stomach were sectioned below the incisura angularis to gain access to the lesser sac and the posterior aspect of the stomach. Subsequently, the stomach was horizontally sectioned with a stapler (Endo GIA™ Roticulator Tri-staple 45 mm-3.5 mm; COVIDIEN)” and a 36-Fr orogastric tube was positioned in the stomach for calibration.
In the posterior plane of the stomach gastro-pancreatic adhesions were released prior to the vertical section of the stomach up to the angle of His.
Therefore, a large gastric pouch was achieved, approximately 18 to 20 cm in length, which was well vascularized.
The previously measured intestine was mobilized in the antecolic and antegastric positions to suture the gastric reservoir to the intestine, which is known in the OAGB technique as an anti-reflux mechanism. The lengths of the excluded biliopancreatic loop and the common loop were measured with a 20/80 ratio, respectively. The jejunal loop was attached for a length of 10 cm to the staple line of the gastric pouch. Enterotomy and gastrotomy procedures were performed with an ultrasonic device of 3 to 4 mm, and 80-90 % of the stapler was inserted (Endo GIA™ Roticulator Tri-staple 45 mm 3.5mm; COVIDIEN) to create a side-to-side gastro-jejunal anastomosis 3.5-4 cm in length.
The anterior plane of the anastomosis was closed using separate stiches with No. 2-0 monocryl suture. The biliopancreatic loop was sutured in the ascending position of the excluded stomach. A pneumatic test was performed on the anastomosis with methylene blue to rule out leakage. A closed drain was placed for 48 hours.
Postoperative evolution: a contrast-enhanced radiographic control study of the gastrointestinal tract was performed in all patients at 24 hours to confirm the absence of leakage or anastomotic stenosis. In all cases, the patients had a liquid diet in the first week, then progressed to a semi-liquid diet during the second week; in the following three weeks a semi-solid diet was consumed, and at the sixth week a diet consisting of solids was started. Every step under careful monitoring.
Insulin doses and oral hypoglycemic agents were systematically modified according to requirements, adjusted to pre- and post-prandial glucose levels for each individual patient.
During follow-up visits at 1 week, 3, 6, 12, and 24 months after surgery, weight, BMI, fasting serum glucose, and HbA1c levels were measured, along with interrogation about the use of insulin and oral hypoglycemic agents to allow pertinent modifications.
Patients were evaluated with various consensus criteria to determine DM2 remission. The most commonly used criteria are those issued by the Spanish Society of Obesity Surgery (SECO), the Spanish Diabetes Society (SED), the Spanish Society for the Study of Obesity (SEEDO), the Spanish Society of Endocrinology and Nutrition (SEEN) in 2013, and the American Diabetes Association (ADA) in 2009. Complete disease remission was considered when HbA1c < 6.5 % or HbA1c < 6 %, respectively, at 12 months after surgery and without pharmaceutical treatment (13) (Table I).
RESULTS
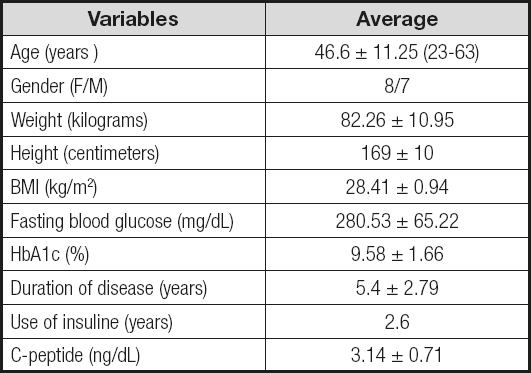
A total of 15 patients were included, 8 women (53.33 %) and 7 men (46.6 %), with a mean age of 46.6 ± 11.25 years.
Mean preoperative weight was 82.26 ± 10.95 kg and BMI was 28.41 ± 0.94 kg/m2; the lowest BMI was 26.4 kg/m2.
One hundred percent of the patients had been diagnosed with DM2 for a mean duration of 5.4 ± 2.79 years, and a mean time under insulin treatment of 2.6 years.
Preoperative C-peptide serum levels had a mean of 3.14 ± 0.71 ng/mL. Mean presurgical fasting glucose levels were 288.53 ± 65.22 mg/dL, and mean preoperative HbA1c was 9.58 ± 1.66 % (Table II).
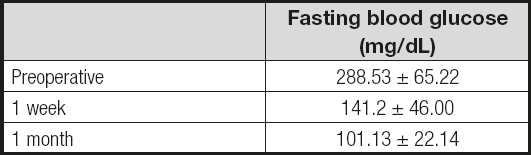
Fasting glucose decreased after the first postoperative week with a mean of 141.2 ± 46, and then 101.13 ± 22.14 within the first month (Table III). After 2 years of surgery mean BMI was 21.8 ± 0.89 kg/m2 and HbA1c was 5.21 ± 0.26 %. Remission rate for DM2 was 100 %. All patients maintained a normal BMI range (Table IV).
DISCUSSION
Epidemiological data confirm the value of early weight loss in DM2. In a study by Lean et al., an increase in survival from 3 to 4 months, weight loss of 10 kg, was associated with a restoration of life expectancy by 35% (14). A planned moderate weight loss of around 10 kg was able to reduce mortality in diabetics by around 25 % in a study by Williamson (15).
Bariatric/metabolic surgery improves control of DM2 through both weight-dependent and weight-independent mechanisms (16).
The gastrointestinal tract is an important contributor to normal glucose homeostasis, and mounting evidence, especially over the past decade, has demonstrated the benefits of bariatric/metabolic surgery to treat and prevent DM2. Some operations engage mechanisms that improve glucose homeostasis independently of weight loss, such as changes in gut hormones, bile acid metabolism, microbiota, and/or intestinal glucose metabolism. In this way sustained favorable effects on blood glucose are achieved for up to 20 years in an observational study, although benefits can decrease over time, with or without weight regain (9).
A striking feature of bariatric/metabolic surgery is the rapid improvement in glycemic control. It is known that many patients with insulin medication prior to surgery will not require it at the time of discharge (16).
The benefits of surgery go beyond the secretion of incretins, and there are other factors that also influence blood glucose improvement regardless of weight loss. The incretin effect or theory of the distal intestine is the mechanism of action of the best known metabolic surgery; it is postulated that the rapid arrival of poorly digested foods to the distal intestine promotes an increase in the secretion of intestinal hormones called incretins, such as GLP-1, which increases insulin secretion and decreases insulin resistance. Over the years it was seen that the incretin effect is only a link in the resolution of the disease. Another explanation is given by the so called theory of the proximal intestine, which is based on a contrary affirmation with the existence of anti-incretin, suggesting the existence of some peptide with an antagonistic effect on incretins; these peptides X are generated in the duodenum. Therefore, an overproduction of anti-incretin could stimulate the factors that cause DM2 and, for this reason, duodenal exclusion techniques for controlling the anti-incretin effect explain the improvement in blood glucose; however, such molecules or peptides have not been identified yet.
The bile is also important for glucose regulation. The alteration of the intestinal flow that derivative techniques provide changes the normal circulation of bile and therefore modifies the reabsorption of bile acids, increasing serum bile acids in the circulation. They suppress the expression of multiple genes involved in hepatic gluconeogenesis, and consequently result in low glucose levels; on the other hand, serum bile acids induce the secretion of incretins directly in the distal intestine.
Bile is not only relevant because of the content of bile acids but also of sodium, since glucose needs sodium to enter the enterocyte and, when the anatomy is modified by a gastric bypass, biliary sodium flow is excluded from a part of intestinal transit, and therefore a decrease in glucose absorption results.
The microbiota also contributes to metabolic control. There is a hypothesis that if a person is fed a fat-rich diet, the proportion of endotoxin-producing bacteria may increase, generating a chronic inflammatory state that induces insulin resistance. When this type of diet is changed after a bariatric procedure, these endotoxins decrease and so does resistance to insulin (17).
The small intestine also contributes to glucose synthesis through a process called intestinal gluconeogenesis. This mechanism involves glucose -6-phosphate synthetase (G6P-ASA) and carbokinase phosphoenolpyruvate (PEPCK), enzymes that are at high concentrations in the liver but not in the normal small intestine; however, after a gastrointestinal surgical rearrangement, a marked elevation of both enzymes has been observed in jejunual and ileal segments. The release of intestinal glucose to portal circulation can be interpreted in hepatic receptors such as glucose from food, thus altering regulatory signals of liver glyconeogenesis (18).
Intestinal adaptation in patients who underwent gastric bypass presents as hyperplasia and hypertrophy of the intestinal mucosa, but having a caloric deficit a compensatory mechanism is activated that consists of a restructuring of glucose conveyors by means of conveyor GLUT-1 that are normally not found in the intestine; however, their expression increases in these patients leading intestinal cells, for their maintenance, to remove glucose from the circulation through these passive conveyors that do not require energy. In this way a decrease occurs in glycemia (18,19).
Clinical trials reveal that bariatric surgery induces the remission of diabetes in 33-90 % of patients at one year after treatment, compared with 0-39 % of those receiving medical treatment. Remission rates may decrease over time but still remain higher in surgically treated patients (16).
Currently, only 1 % of the patients who meet the criteria for metabolic surgery actually undergo surgery. Metabolic surgery is a vastly underutilized tool for the treatment and/or cure of DM2. This unfortunate fact may be due to the misconception that metabolic surgery is associated with increased rates of postoperative complications. However, it has been shown that the morbidity and mortality associated with metabolic surgery has experienced a constant decline due to the widespread application of minimally invasive techniques and the formalization of national quality programs (10).
The DSS II delegates identified that there are areas for future research related to metabolic surgery, such as the development and evaluation of criteria for surgery that are more appropriate than BMI alone in people with DM2 (9).
Also, this may determine which operation is the optimal option for each individual patient. Currently, the DSS II only proposes 4 procedures for metabolic surgery: laparoscopic adjustable gastric banding, Roux-en-Y gastric bypass, vertical sleeve gastrectomy, and biliopancreatic diversion (9). However, more recently IFSO recognizes OAGB as a bariatric/metabolic procedure (12). Studies have reported data over the very long term (> 5 years) about the treatment of DM2 and recurrence of disease after OAGB. A study confirmed that OAGB is a powerful and durable therapy for the treatment of DM2 - the DM2 control rate was 76.1 % short-term, and 64.2 % long-term (20).
Several studies have shown similar or even higher weight loss, and resolution of obesity-related comorbidities with OAGB as compared to RYGB, but there are few comparative studies of these 2 procedures long-term. In a retrospective study with a 5-year follow-up the resolution rates for DM2 (79 %), systemic arterial hypertension (56 %), obstructive sleep apnea (> 90 %), and dyslipidemia (56 %) were higher in the OAGB group (21).
In a comparative study between OAGB and Roux-en-Y banded gastric bypass (BGBP), the resolution of DM2 at 5 years was 79.16 % and 71.42 % in the OAGB and BGBP groups, respectively, which demonstrates the good results of OAGB in the long term (22).
OAGB-MGB and RYGB comparative studies are understandably rare. To date, WJ Lee and M. Robert have published only two RCTs. At present, in most centers there is no authentic intra-institutional approach adapted to bariatric surgery, so a single-institution study compared a large number of patients for a short period of treatment with these two highly effective surgical procedures. They found that partial or total remission of individual comorbidities for both procedures was between 79.55 % and 100.00 % without any important changes at 1, 2, and 3 years (23).
In the study conducted in 415 patients who were included in the database of the European Council of Accreditation of Excellence Centers for Bariatric Surgery (EAC-BS), and who underwent OAGB, 79 patients were selected with altered glycemic levels in the preoperative blood sample, 47 pre-diabetic patients and 32 diabetics. Presurgical BMI was 42.73 kg/m2 in prediabetics and 43.19 kg/m2 in diabetics.
The conclusions of this study showed that OAGB was an effective surgical technique for weight loss in prediabetic and diabetic patients after one year of follow-up.
Blood glucose is normalized in prediabetic patients since 100 % solve their prediabetic state within the first month after surgery. In the diabetic group, in the 3rd month after surgery diabetes had resolved in all evaluated patients, with normal levels of blood glucose and HbA1c, and total absence of medications.
The best results in both weight loss and blood glucose levels were observed in the prediabetic patients, which is suggested to anticipate the damage that obesity and associated comorbidities cause over time, hence supporting bariatric surgery as soon as possible (24).
Numerous studies have already been published using the Roux-en-Y gastric bypass technique in patients with BMI < 35 kg/m2. This surgical technique has shown good results and its safety has been demonstrated in mildly obese patients (25); however, in our series we performed one-anastomosis gastric bypass with adequate results in terms of DM2 remission without increasing risks or complications. One-anastomosis gastric bypass (OAGB) has multiple advantages over conventional Roux-en-Y gastric bypass since the alimentary loop is eliminated and therefore the second anastomosis is suppressed. The internal hernias and potential complications related to the jejuno-jejunal anastomosis disappear, the direct endoscopic access to the only anastomosis between the stomach and intestine is maintained, and fistula formation and leak rates are much lower due to a greater blood supply to the gastric reservoir and the fact that neither the intestine nor the mesentery are divided, so that the blood supply to the anastomosis remains at its possible maximum (26).
OAGB is superior to RYGB as a solution for insufficient weight loss and weight regain after failed restrictive surgery, providing more weight loss and a lower early complications rate (27).
Although one-anastomosis gastric bypass is not recognized by the DSS-II in the diabetes mellitus type 2 treatment algorithm, there is evidence supported by IFSO of its effectiveness versus other procedures (12).
The Declaration of the IFSO Consensus Conference (International Federation of Obesity Surgery and Metabolic Disorders) on One-Anastomosis Gastric Bypass (OAGB-MGB) considered OAGB-MGB suitable for patients with a BMI of 25-30 kg/m2 and diabetes; however, Delphi studies, by their nature, are not designed to measure truth but the degree of consensus between different opinions, so their results are considered level-IV evidence (28).
There are only two series with non-obese patients in whom OAGB technique was used. In the first series García Caballero performed a gastric pouch twice the size that is normally performed for a patient with obesity; on the other hand, the anastomosis was at 100 cm from the Treitz ligament, without measuring the entire intestine (29). In the second series, published by Kim Z, a vertical gastric reservoir of 150-180 cc was formed, and the distance from the anastomosis was 200 cm (30). In our series the size of the gastric reservoir was exactly as described in the original technique by Dr. Miguel Ángel Carbajo (31). We modified the distance to which the anastomosis would be placed by measuring the entire intestine, and then a 20/80 ratio was determined for the excluded biliopancreatic loop and common loop, respectively.
In the series by García Caballero mean age was 63 years (29). In Kim's study the sample was younger with a mean age of 46.9 years (30), similar to our population (mean age, 46.6 years). This is relevant since young age is one of the positive predictors for good outcomes. Another difference found between the series by García Caballero (29), Kim (30), and our team is mean DM2 duration which was reported as 16.9 years, 6.6 years, and 5.4 years, respectively, this being another factor that influences the results of metabolic surgery. Another important parameter to consider is mean C-peptide levels - García Caballero (29) reported 1.65 ng/dL (29), Kim 2.78 ng/dL (30), and our team 3.14 ng/dL.
Mean preoperative BMI in the series by García Caballero was 26.9 kg/m2 (29), in the series by Kim 27.2 kg/m2 (30), and in ours 28.41 kg/m2, which is closer to grade-I obesity. Regarding metabolic parameters, García Caballero reported a decrease in fasting glycemia from 203 mg/dL preoperatively to 100 mg/ dL at 6 months postoperatively. HbA1c decreased from 8.3 % preoperatively to 6.6 % at 6 months.
Kim reported a preoperative fasting glucose of 222 mg/dL, and then 144 mg/dL at 6 months postoperatively, with HbA1c going down from 9.7 % to 6.7 % pre- and post-operatively at 6 months, respectively (30).
In our series, mean preoperative fasting glycemia was 288 mg/dL, and 90 mg/dL at 6 months postoperatively; HbA1c diminished from 9.58 % to 5.48 % pre- and post-operatively at 6 months, respectively. Up to this point the improvement seen in our study was greater since fasting blood glucose levels diminished almost twice as much when compared to the above series, probably because of the distance at which the anastomosis was placed, and the 20/80 ratio proposed in our study. In addition, we continued follow-up to 12 and 24 months, obtaining fast blood glucose levels of 87 mg/dL and 94 mg/dL, respectively, and HbA1c of 5.03 % and 5.21 %, respectively. According to these data, our patients are in complete remission for DM2, both according to the criteria established by the European Associations in 2013 (SEEN, SECO, SEEDO, SED), and the ADA in 2009 (13).
There are other studies where one-anastomosis gastric bypass was used but in patients with BMI < 35 kg/m2. Navarrete reported a series of 16 patients with a mean age of 42.9 years (32). It was more similar to our series with a mean age of 46.6 years. Mean BMI was 32.2 kg/ m2, and in our case was 28.41 kg/m2. Navarrete's series reported a fasting blood glucose of 193.6 mg/dL with a decrease to 78.8 mg/dL postoperatively at 12 months (32). In our series, fasting blood glucose decreased from 288 mg/dL preoperatively to 87 mg/ dL at 12 months; on the other hand, Navarrete's HbA1c was 8.4 % preoperatively and 6.1 % postoperatively at 12 months (32), and ours was 9.58 % and then 5.03 % at 12 months. At this point, both series present complete DM2 remission in terms of the SEEN, SECO, SEEDO, SED criteria established in 2013 (13). In Navarrete's series (32) no levels of C-peptide were reported, nor was the evolution time of DM2. Regarding the surgical technique, they performed a gastric pouch, as we did, with the original technique of Dr. Miguel Ángel Carbajo (31), but they performed the anastomosis at 150 cm from the Treitz ligament without considering the entire intestine (32) whereas we used the 20/80 ratio as previously commented.
It is possible to corroborate with these results what has already been reported in the literature regarding the best postoperative results of metabolic surgery, which is more effective the younger the patients are, the shorter disease duration is, and the better the pancreatic reserve.
None of the series of non-obese diabetic patients or of patients with obesity grade 1 have a follow-up longer than 1 year.
Our series has a 2-year follow-up, where it can be observed that the levels of fasting glucose and HbA1c still remain within normal ranges but begin to increase, so it would be advisable to have a long-term follow-up of these patients to corroborate whether the distance at which we performed the anastomosis is adequate, since it should be taken into account that DM2 and obesity are chronic diseases, and long-term control in younger patients may require an adjustment of the intestinal loop with a 30/70 or 35/65 ratio for the biliopancreatic and common loop, respectively.
CONCLUSIONS
The treatment of DM2 is far from being effective using a medical therapy and lifestyle modifications approach alone, and metabolic surgery is a tool that significantly improves morbidity and mortality in these patients.
OAGB is a bariatric/metabolic procedure accepted by the international scientific community, and currently represents the third most commonly performed bariatric surgical technique in the world, maintaining a different metabolic profile, much higher than that of RYGB, as has been demonstrated in multiple comparative studies, so we consider that the societies and federations responsible for promoting a DM2 treatment algorithm should consider this procedure too.
On the other hand, the benefits of metabolic surgery should be used for patients without obesity, since being overweight indicates excess fat, a condition that can decrease with the procedure without causing any degree of malnutrition, since surgery can be carried out following an individualized approach, determining the site of the anastomosis depending on the entire small intestine of each patient. In this series we obtained complete DM2 remissions and all patients had a normal BMI at 12 and 24 months; however, longer-term studies are needed to establish which is the appropriate proportion for biliopancreatic and common handles, in which a remission of disease without weight loss greater than necessary is maintained.