INTRODUCTION
Dysphagia is a broad term referring to difficulty with moving a food bolus from the oral cavity to the stomach. Dysphagia affects patients of all ages and may be a symptom of several diseases. Anatomically, dysphagia is commonly classified as either oropharyngeal (accounting for approximately 80 % of diagnosed cases) or esophageal (about 20 %) (1). From a pathophysiological point of view, dysphagia can occur due to structural alterations (mechanical or obstructive dysphagia) or as a consequence of functional alterations (neurogenic dysphagia). Clinically, dysphagia due to structural alterations predominantly presents as difficulty eating solid foods, while dysphagia due to functional alterations predominantly involves difficulty swallowing liquids (1,2). Age may adversely affect the swallowing process due to changes in the structure, motility, coordination, and sensitivity of the anatomical structures involved in swallowing. Additionally, decreased saliva production and tooth loss may lead to impaired ability to handle a food bolus; the presence of this alteration in the healthy elderly population is referred to as presbyphagia (3).
The intake of both liquids and solid foods is compromised in dysphagia patients, thus hydration and nutritional status may be affected. Dysphagia has been identified as a risk factor for the development of malnutrition due to the combination of insufficient oral intake, low nutrient density of modified texture diets, and increased nutritional requirements underlying the disease process (4-6). In elderly patients dehydration is associated with worse prognosis, higher mortality, and higher healthcare expenditure, and is involved in multiple pathological processes such as kidney failure, cardiovascular decompensation, constipation, and increased pharmacological toxicity (7). Of note, dysphagia also leads to changes in the texture of food and drinks, thus altering the sensory characteristics of the substance and, potentially, causing them to be rejected by a patient, thereby further increasing the risk of malnutrition and dehydration due to low intake.
Once dysphagia is detected, it is important to carry out an assessment of the patient's nutritional status, and adapt their oral diet accordingly. Adaptations to food texture must be carried out on an individual basis, according to the characteristics and severity of the dysphagia. Tests such as the viscosity volume test have been shown to be effective in determining the texture and volume of food that can safely and effectively be swallowed by a patient (8). These textures must be standardised, which is the objective of the International Dysphagia Diet Standarization Initiative (www.iddsi.org). Several types of thickeners have been developed to achieve proper viscosity of various liquids and increase the safety of swallowing them. There is wide evidence that, if viscosity is adequately adapted to the characteristics of a patient, changing the texture of the diet and thickening liquids increases the safety of swallowing and reduces aspirations (9-13).
Thickeners are substances with the ability to retain water. Currently, there are several types of thickeners on the market. Starch-based thickeners can be used in any liquid (water, milk, juices, etc).; however, the resulting solution may have a cloudy appearance with a grainy texture, and viscosity continues to increase over time as more water is absorbed (14). Additionally, starch must be modified by applying various technological treatments to avoid hydrolysis by saliva.
In recent years a new generation of thickeners have been developed that are based on gums rather than starch, although sometimes these are combined. Several ingredients are used in these thickeners: guar gum obtained from Cyamopsis tetragonolobus; xanthan gum, which is produced during the fermentation of corn by the bacterium Xanthomonas campestris; tara gum, derived from the endosperm of the seeds of Caesalpinia spinosa; and carrageenans obtained from algae. Among these thickeners, xanthan gum stands out for its thickening, emulsifying, stabilizing, and foaming properties that provide high viscosity at low concentrations. Xanthan gum is soluble in both hot and cold liquids, is stable to acidity, and resists freezing and thawing (15).
Recent efforts in medical science have been dedicated to the study of thickeners from a rheological point of view. However, there is a scarcity of works regarding the acceptability of thickened fluids. Studies are needed that examine the acceptance of the various thickeners available from the sensorial point of view of the patients. A previous study showed that dysphagia patients perceive thickened beverages as less palatable than their liquid versions (16). Acceptance of a thickener can be highly variable depending on factors such as composition, the flavor of the thickened drink, and the texture achieved (17-19). On the other hand, the addition of a thickener modifies the flavor of the drink, often attenuating it (20).
A pilot study was designed to assess the acceptance of several types of thickeners in patients with oropharyngeal dysphagia, along with the effects of adding a food flavoring to the thickened water. Secondary aims were to evaluate the effects of various thickeners, as well as the addition of flavoring, on the sensory characteristics of the samples, and to evaluate these effects on patients’ oral intake of liquid.
MATERIAL AND METHODS
A randomized, controlled, pilot study of nutritional intervention was designed that included four different groups: modified-starch thickener without flavoring, gum-based thickener without flavoring, modified-starch thickener with flavoring, and gum-based thickener with flavoring. The study was carried out between January and March 2020 in the hospitalization wards of the Complexo Hospitalario Universitario Hospital A Coruña (CHUAC), a university hospital with 1,415 beds installed for a reference population of 505,797 people. The present study was carried out in accordance with the Declaration of Helsinki of the World Medical Association and the ratifications of its following assemblies on ethical principles for medical research in human beings, and the Convention related to human rights and biomedicine, enacted in Oviedo on April 4, 1997, with successive updates. The research protocol was approved by the Research Ethics Committee of the CHUAC and was registered in Clinical Trials (ref. no. NCT 04305860).
The inclusion criteria were patients older than 18 years with a previous diagnosis of oropharyngeal dysphagia. Hospitalized patients receiving the center's dysphagia diet were sought for recruitment. This diet is characterized by offering pureed foods with a homogeneous texture and thickened liquids. In all patients invited to participate, the medical history was reviewed to confirm the previous diagnosis of oropharyngeal dysphagia and the previous use of thickeners. No procedure was done to confirm the diagnosis of dysphagia. Exclusion criteria included expected hospital stay of less than 24 hours, allergy to any ingredient of the thickeners or flavorings, any cognitive impairment that might prevent the sensory evaluation of the assessed thickeners, and life expectancy limited by terminal illnesses. Recruitment was performed consecutively, upon request for consent, from patients admitted to acute hospitalization wards of the CHUAC. Patients admitted during the weekend or non-working days were recruited the following working day.
The study was conducted in 40 patients. As there was no scientific evidence available to calculate the sample size, a pilot study was designed. According to the literature, in pilot studies it is recommended to include between 30 and 50 participants (21). Patients were randomized into the above-mentioned four groups in a 1:1:1:1 ratio, using Epidat 3.1® (Consellería de Sanidade, Xunta de Galicia, in collaboration with Organización Panamericana de la Salud).
For flavoring, the patients in the group receiving modified starch were administered Bi1 Espesante®, while the group receiving the gum-based thickener were given Bi1 Clear® (Adventia Healthcare S.A).. The flavoring was performed by adding 5 drops of Bi1 Aromas to each glass of thickened liquid. The patient chose one among the available flavors (melon, strawberry, lemon, orange, or grape). Thickeners were used according to the manufacturer's recommendations, adapting the texture to the patient's needs.
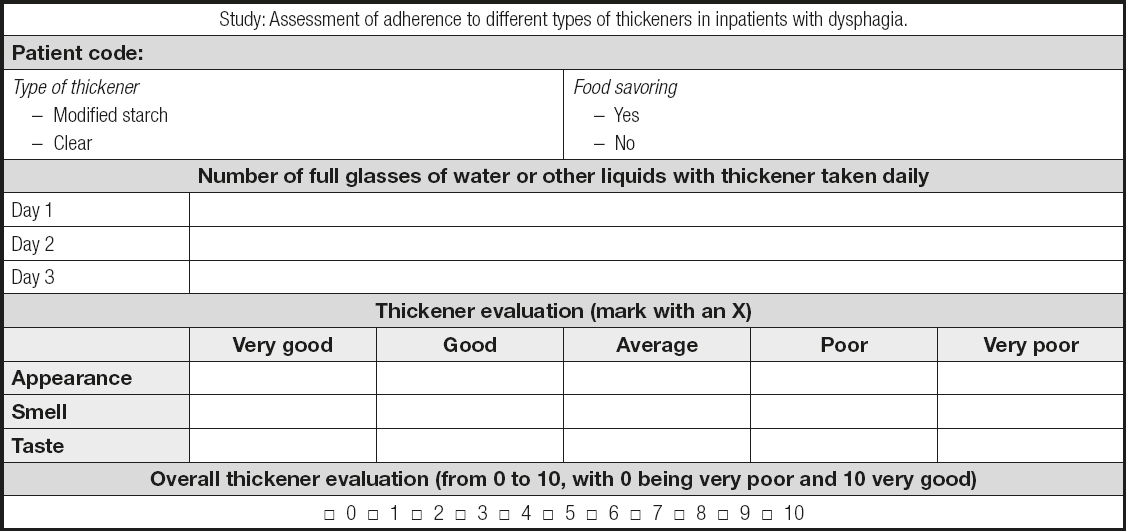
Since there are no validated questionnaires in Spanish to assess the sensory characteristics and acceptance of thickened liquids, a questionnaire was developed for the current study. In preparing this questionnaire, the sensory characteristics to be assessed were considered, as well as the global assessment of the product and the volume of liquid ingested. Each item and its corresponding score were established by consensus among the researchers. As this was a pilot study, the questionnaire was not validated. The questionnaire included an assessment of the sensory characteristics of thickened liquids, such as appearance, smell, and taste, on a scale from very good (5 points) to very bad (1 point), as well as a global rating of the thickener (scored from 0 to 10, with 0 being very bad and 10 being very good). A total value was calculated by adding the scores of the evaluated items. Patients were asked to record the number of glasses of water, or any other liquid, that they consumed during a period of three consecutive days to assess the total amount ingested. Parameters such as age, sex, and diagnosis were obtained from the subjects’ electronic medical records (Anex I).
Categorical data were summarized using percentages, and compared using the Chi-square test. Quantitative data were summarized with means and standard deviation (SD), and analyzed using Student's t-test for independent measures (when comparing two groups) or the analysis of variance (ANOVA) test (when three or more than three groups were compared). A p-value lower than 0.05 was considered significant.
RESULTS
A sample of 40 patients was recruited, of which 20 were women. The average age of the sample was 79.1 (12.4) years. The main diagnostic categories were neurological diseases (45.0 %), infectious diseases (25.0 %), cardiovascular diseases (10.0 %), oncological diseases (7.5 %), and other (12.5 %). These characteristics were similar among the experimental groups (Table I).
Table I. Comparison of the main characteristics of patients in the sample
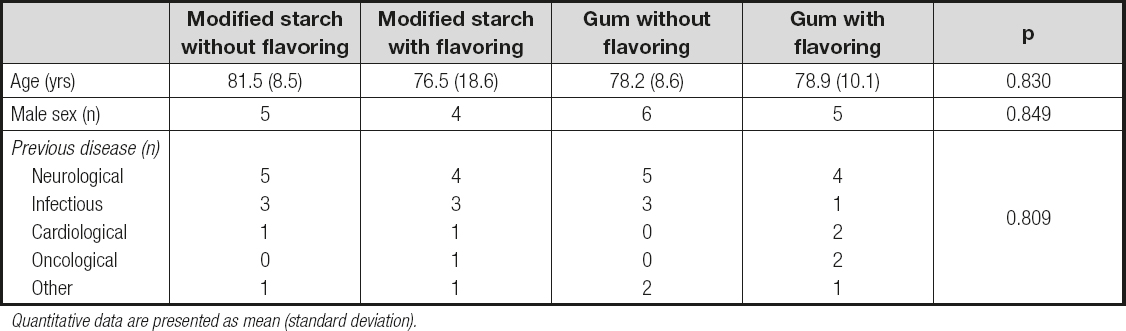
Quantitative data are presented as mean (standard deviation).
The gum-based thickener received a higher sensory score (Table II). The global rating score was 5.10 (2.43) for modified starch and 7.45 (1.57) for the gum-based thickener; these values were significantly different (p = 0.001). The total score was also significantly higher (p < 0.001) for the gum thickener as compared to modified starch, with values of 19.75 (3.08) vs. 13.60 (5.69), respectively. Additionally, the increased appreciation of gum-based thickeners was associated with a higher intake of water. The addition of a flavoring was associated with better sensory perception of the thickener (with the exception of appearance), which also resulted in higher fluid consumption (Table III). The comparisons between the four experimental groups is summarized in table IV.
Table II. Comparison of the two types of thickeners
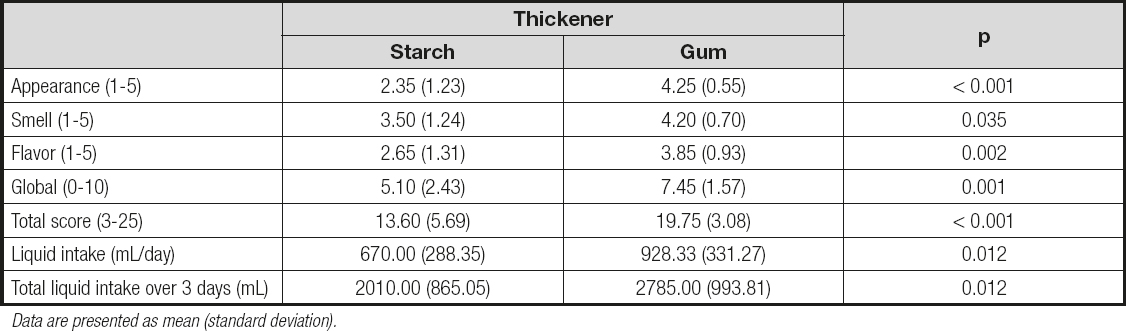
Data are presented as mean (standard deviation).
Table III. Effects of the addition of flavoring on sensory perception of the liquid and total liquid intake
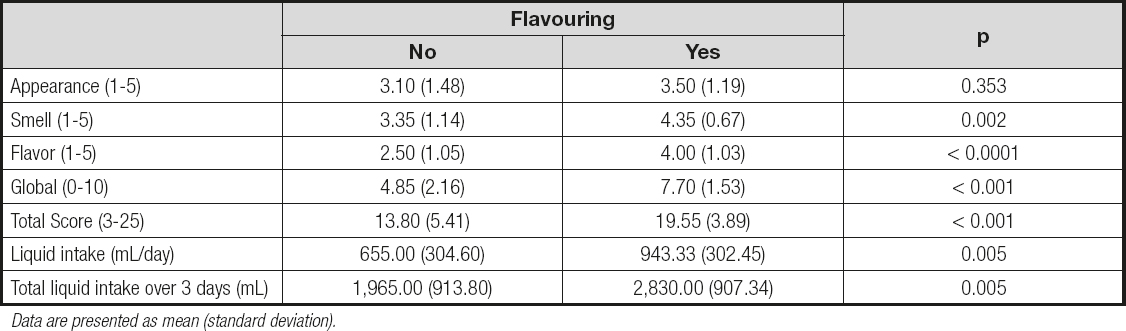
Data are presented as mean (standard deviation).
Table IV. Comparison of the four experimental groups
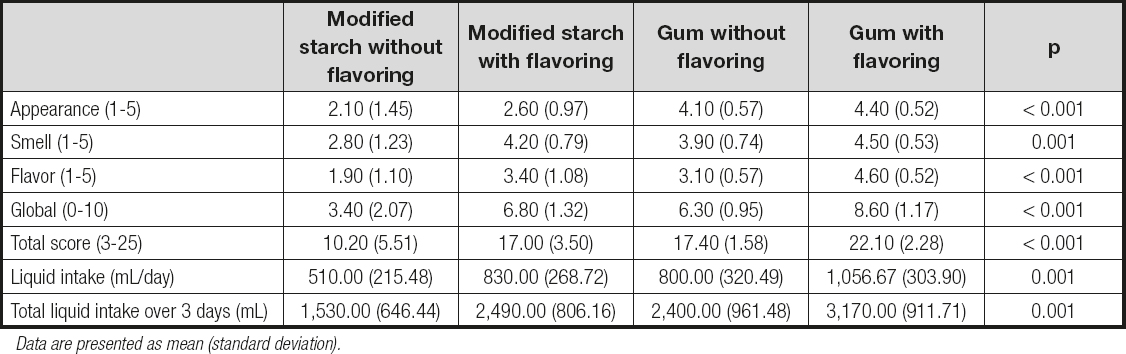
Data are presented as mean (standard deviation).
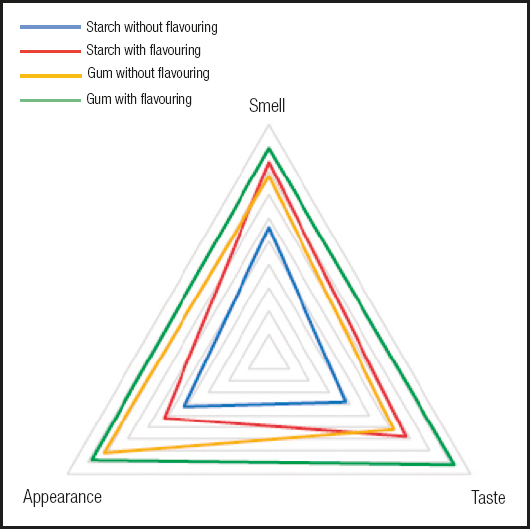
Finally, figure 1 shows the results obtained from the sensory evaluation performed by each experimental group. It may be seen that the water thickened with the gum-based thickener had a better appearance, while the modified-starch thickener without flavoring was evaluated the worst in each of the scored items.
DISCUSSION
In this study we showed that gum-based thickeners are more valued by patients than starch-based thickeners in terms of their sensory characteristics (appearance, smell, and taste). Additionally, patients who received clear thickeners consumed a greater volume of water throughout the day when compared to those who received modified-starch thickeners. Our results also demonstrate that thickened water is better accepted, and water intake is significantly higher, when a food flavoring is added to the thickener.
Thickeners based on modified starch have several characteristics that may explain their lower acceptance by patients. For example, these thickeners provide a floury taste and have poor stability, with a viscosity that increases over time. Furthermore, modified starch-based thickeners have poor solubility, therefore liquids become cloudy in appearance and acquire a grainy texture. There were no changes in the evaluation of their appearance when a flavoring was added, as long as it did not modify the granulated texture of the solution.
However, when comparing starch-based and gum-based thickeners, there was a significant difference in the assessment of their appearance. This can be explained by the lower formation of lumps with gums, since these thickeners dissolve more easily and require less product to achieve the desired texture, resulting in a more natural appearance of the drink. Furthermore, the viscosity of gum-based thickeners is more stable and durable over time (22). The differences in the taste evaluation were also significant, as gums modify this attribute to a lesser extent than starch.
Sensory characteristics are decisive in the acceptability of solid and liquid foods by patients and, when the texture of liquids is modified using thickeners, these characteristics are modified, potentially resulting in patient rejection and a decrease in intake leading to an increased risk of dehydration (23,24). In the case of liquids, the more the viscosity is increased in relation to the original liquid, the greater the rejection. Additionally, thickeners may increase the sensations of satiety and thirst. For both reasons, i.e., the lower palatability of thickened liquids and their reduced capacity to quench thirst, patients who consume liquids with thickener often drink less than those consuming liquids without a thickener (25). This is reflected by the data obtained in this study, since fluid intake was significantly higher when clear thickeners were used and flavoring was added. The combination of both aspects in a clear, flavored thickener was seen as the best valued option, resulting in the highest fluid intake among the four study options. Meanwhile, the non-flavored starch-based thickener presented the worst rating and resulted in the lowest fluid intake.
While modified-starch thickens liquids as the starch molecules swell, gum-based thickeners do so by creating a mesh in which the water molecules become trapped. The latter method provides several theoretical advantages, including requiring less product to achieve a desired texture (which preserves both appearance and taste), stable and durable viscosity over time, and easier dissolution in water with decreased lump formation. Studies using modified starch-based thickeners have shown reductions in tracheobronchial aspirations and aspiration pneumonia, the mechanism of action of which has been attributed to the slower speed at which liquids pass through the pharynx. Additionally, in patients with poor bolus propulsion such as the elderly or those with neurodegenerative diseases, it was observed that increased viscosity resulted in increased residue in the oropharynx after swallowing (26). Xanthan gum thickeners, such as those used in this study, improve swallowing safety without increasing oropharyngeal residue; this difference may modify a patient's perception of the product.
The interest of this study lies in the absence of similar prior studies, in which several types of thickener are compared from a sensory point of view while analyzing how these characteristics influence fluid intake, along with the possibility of improving intake via the addition of food flavoring. In any case, this study has some limitations. First, masking was not possible as, in most cases, the patients added the thickener to their liquids themselves, or had a family member do it, and the presentations of each were clearly differentiable. However, the data analysis was performed in a masked way. The recording of fluid intake amount during the three-day period, which was performed by the patients and/or their relatives, may be biased by observation. Neither the thickener assessment was independently analyzed according to the type of drink consumed (water vs. other drinks). Additionally, the results may be influenced by the amount of thickener required in each individual case to reach the adequate texture according to the severity of dysphagia. We did not evaluate the effect of the texture achieved on the acceptance of the thickened liquid due to the small sample size. A pilot study was designed as the most operative way to test the hypothesis that the type of thickener and its flavor condition acceptance in hospitalized patients. In the future, the design of a larger study, with a crossover design where patients receive different thickeners, may help to deepen the results obtained in this study.
CONCLUSIONS
The results of this study show that clear gum-based thickeners may be more acceptable to patients with dysphagia than modified starch-based thickeners. The differences in overall assessment and in the assessment of the sensory characteristics (taste, smell, and appearance) of each type of thickener were statistically significant. Acceptance of the thickener was further increased through the addition of a food flavoring, which resulted in increased oral fluid intake during the observation period. These results could help improve adherence in patients with dysphagia to the use of thickeners, and thus contribute to reducing dehydration risk.